TRANS-1,4-CYCLOHEXANEDIMETHANOL | 3236-48-4
中文名称
——
中文别名
——
英文名称
TRANS-1,4-CYCLOHEXANEDIMETHANOL
英文别名
1,4-cyclohexanedimethanol
CAS
3236-48-4
化学式
C8H16O2
mdl
——
分子量
144.214
InChiKey
YIMQCDZDWXUDCA-ZKCHVHJHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:61-66 °C
-
沸点:283 °C
-
密度:1.02
-
闪点:>109℃
-
溶解度:可溶于氯仿、DMSO、甲醇
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):0.78
-
重原子数:10.0
-
可旋转键数:2.0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.46
-
氢给体数:2.0
-
氢受体数:2.0
安全信息
-
安全说明:S26,S39
-
危险类别码:R36
-
海关编码:2906199090
-
危险性防范说明:P305+P351+P338
-
危险性描述:H319
-
储存条件:存放在阴凉干燥处即可。
SDS
反-1,4-环己基二甲醇 修改号码:5
模块 1. 化学品
产品名称: trans-1,4-Cyclohexanedimethanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-1,4-环己基二甲醇
百分比: >98.0%(GC)
CAS编码: 3236-48-4
俗名: trans-1,4-Bis(hydroxymethyl)cyclohexane
分子式: C8H16O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
反-1,4-环己基二甲醇 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
易湿
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
67°C
沸点/沸程 164 °C/1.6kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
反-1,4-环己基二甲醇 修改号码:5
模块 9. 理化特性
[水] 无资料
[其他溶剂]
溶于: 甲醇
自燃温度: 316°C
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
反-1,4-环己基二甲醇 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: trans-1,4-Cyclohexanedimethanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-1,4-环己基二甲醇
百分比: >98.0%(GC)
CAS编码: 3236-48-4
俗名: trans-1,4-Bis(hydroxymethyl)cyclohexane
分子式: C8H16O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
反-1,4-环己基二甲醇 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
易湿
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
67°C
沸点/沸程 164 °C/1.6kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
反-1,4-环己基二甲醇 修改号码:5
模块 9. 理化特性
[水] 无资料
[其他溶剂]
溶于: 甲醇
自燃温度: 316°C
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
反-1,4-环己基二甲醇 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-环己烷二甲醇 bis-1,4-(hydroxymethyl)cyclohexane 105-08-8 C8H16O2 144.214 —— trans-1,4-Cyclohexanedicarboxylic acid 619-82-9 C8H12O4 172.181 1,4-环己烷二甲酸 cyclohexane-1,4-dicarboxylic acid 1076-97-7 C8H12O4 172.181 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,4-环己烷二甲醇 bis-1,4-(hydroxymethyl)cyclohexane 105-08-8 C8H16O2 144.214 —— cis-CHDM 3236-47-3 C8H16O2 144.214 —— ((1r,4r)-4-methylcyclohexyl)methanol 3937-49-3 C8H16O 128.214 —— (1R,3R)-cyclohexane-1,3-diyldimethanol 13022-98-5 C8H16O2 144.214 —— trans-4-[(Z)-prop-1-en-1-yl]cyclohexanemethanol 211995-98-1 C10H18O 154.252
反应信息
-
作为反应物:描述:TRANS-1,4-CYCLOHEXANEDIMETHANOL 在 copper(l) iodide 吡啶 、 咪唑 、 四(三苯基膦)钯 、 TEA 、 四丁基氟化铵 、 三苯基膦 作用下, 以 氯仿 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 12.0h, 生成 Apadenoson参考文献:名称:环AMP依赖性抑制人类嗜中性粒细胞氧化活性的取代2-丙炔基环己基腺苷A(2A)受体激动剂。摘要:在与腺苷受体(ARs)的四个亚型的结合测定中评估了在环己基环的4-位上反式取代的新型2-丙炔基环己基-5'-N:-乙氧基羧酰胺基腺苷。两种酯,4-(3- [6-氨基-9-(5-乙基氨基甲酰基-3,4-二羟基-四氢呋喃-2-基)-9H-嘌呤-2-基]-丙-2-炔基) -环己烷甲酸甲酯(ATL146e)和乙酸4-(3- [6-氨基-9-(5-乙基氨基甲酰基-3,4-二羟基-四氢呋喃-2-基)-9H-嘌呤-2-基对人A(2A)而言,]-丙-2-炔基)-环己基甲基酯(ATL193)的效力比2- [4-(2-羧乙基)苯乙基氨基] -5'-N:-乙基羧酰胺基腺苷(CGS21680)强50倍以上AR绑定。人类A(2A)AR亲和力取代环己基-丙基腺苷腺苷类似物与环己基侧链的极性成反比。对人中性粒细胞[环AMP](i)的A(2A)AR激动剂刺激和对中性粒细胞氧化爆发的抑制作用具有相当的效力顺序。ATL146DOI:10.1038/sj.bjp.0703893
-
作为产物:描述:(E,E)-2,4-己二烯二酸二甲酯 在 甲醇 、 4-二甲氨基吡啶 、 10 wt% Pd(OH)2 on carbon 、 氢气 、 二异丁基氢化铝 、 potassium carbonate 、 三乙胺 作用下, 以 甲醇 、 二氯甲烷 、 乙酸乙酯 、 间二甲苯 、 甲苯 为溶剂, 250.0 ℃ 、10.44 MPa 条件下, 反应 55.15h, 生成 TRANS-1,4-CYCLOHEXANEDIMETHANOL参考文献:名称:Low-pressure synthesis of cyclohexanedimethanol and derivatives摘要:该发明涉及一种制备式(I)的1,4-二取代环己烷化合物的过程: 其中A为—OH、—OR、Br或Cl; R为硅基团、烃基团或含有1至12个碳原子的酰基团。 该过程包括以下步骤:将乙烯与(2E,4E)-己二烯化合物反应,产生3,6-二取代环己-1-烯化合物,然后将3,6-二取代环己-1-烯化合物加氢处理,得到式(I)的1,4-二取代环己烷化合物。公开号:US09115155B1
文献信息
-
[EN] TRICYCLIC MODULATORS OF TNF SIGNALING<br/>[FR] MODULATEURS TRICYCLIQUES DE LA SIGNALISATION DU TNF申请人:ABBVIE INC公开号:WO2016168641A1公开(公告)日:2016-10-20The invention provides tricyclic heterocyclic compounds, pharmaceutically acceptable salts, prodrugs, biologically active metabolites, stereoisomers and isomers thereof wherein the variables are defined herein. The compounds of the invention may be useful for treating immunological and oncological conditions.
-
Substituted Imidazopyridines as HDM2 Inhibitors申请人:Merck Sharp & Dohme Corp.公开号:US20140179680A1公开(公告)日:2014-06-26The present invention provides substituted imidazopyridines as described herein or a pharmaceutically acceptable salt or solvate thereof. The representative compounds are useful as inhibitors of the HDM2 protein. Also disclosed are pharmaceutical compositions comprising the above compounds and potential methods of treating cancer using the same.
-
Synthesis and pharmacological characterization of novel N -( trans -4-(2-(4-(benzo[ d ]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)amides as potential multireceptor atypical antipsychotics作者:Xiao-Wen Chen、Yuan-Yuan Sun、Lei Fu、Jian-Qi LiDOI:10.1016/j.ejmech.2016.07.038日期:2016.11benzisothiazolylpiperazine derivatives combining potent dopamine D2 and D3, and serotonin 5-HT1A and 5-HT2A receptor properties were synthesized and evaluated for their potential antipsychotic properties. The most-promising derivative was 9j. The unique pharmacological features of 9j were a high affinity for D2, D3, 5-HT1A, and 5-HT2A receptors, together with a 20-fold selectivity for the D3 versus D2 subtype, and合成了一系列结合强力多巴胺D 2和D 3以及5-羟色胺5-HT 1A和5-HT 2A受体特性的新型苯并异噻唑基哌嗪衍生物,并评估了其潜在的抗精神病特性。最有前途的导数是9j。的独特的药理学特征9J是为d的高亲和性2,d 3,5-HT 1A和5-HT 2A受体,与用于d 20倍的选择性一起3与d 2亚型,和对于低亲和力毒蕈碱M 1(降低抗胆碱能副作用的风险)和hERG通道(降低QT间期延长的发生率)。在动物行为模型中,9j抑制了苯环利定的运动刺激作用,阻断了条件回避反应,并改善了大鼠的新型物体识别测试中的认知缺陷。9j表现出较低的僵化潜能,与利培酮的结果一致。另外,在大鼠中检测到了良好的9j脑渗透率。这些研究表明9j是潜在的非典型抗精神病药候选物。
-
Light‐Promoted Nickel Catalysis: Etherification of Aryl Electrophiles with Alcohols Catalyzed by a Ni <sup>II</sup> ‐Aryl Complex作者:Liu Yang、Huan‐Huan Lu、Chu‐Hui Lai、Gang Li、Wei Zhang、Rui Cao、Fengyi Liu、Chao Wang、Jianliang Xiao、Dong XueDOI:10.1002/anie.202003359日期:2020.7.27highly effective C−O coupling reaction of (hetero)aryl electrophiles with primary and secondary alcohols is reported. Catalyzed by a NiII‐aryl complex under long‐wave UV (390–395 nm) irradiation in the presence of a soluble amine base without any additional photosensitizer, the reaction enables the etherification of aryl bromides and aryl chlorides as well as sulfonates with a wide range of primary
-
Design, Synthesis, and Evaluation of Novel A<sub>2A</sub> Adenosine Receptor Agonists作者:Jayson M. Rieger、Milton L. Brown、Gail W. Sullivan、Joel Linden、Timothy L. MacdonaldDOI:10.1021/jm0003642日期:2001.2.1We have been interested in the design, synthesis, and evaluation of novel adenosine A2A agonists. Through the use of comparative molecular field analysis (CoMFA) we have generated a training model that includes 78 structurally diverse A2A agonists and correlated their affinity for isolated rat brain receptors with differences in their structural and electrostatic properties. We validated this model
表征谱图
-
氢谱1HNMR
-
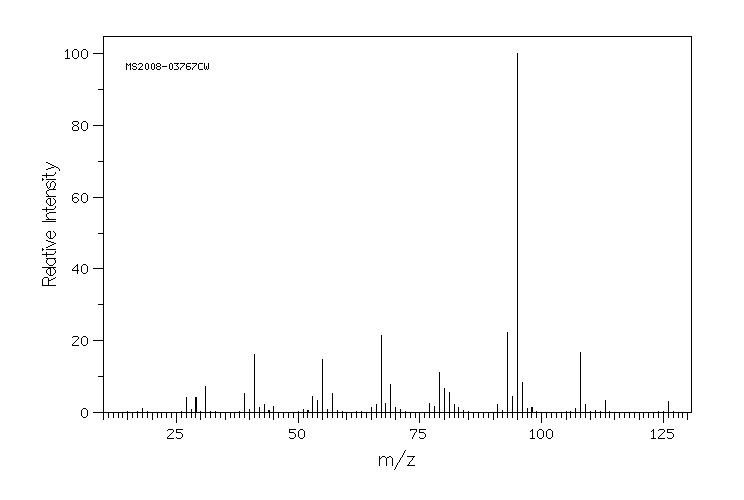
质谱MS
-
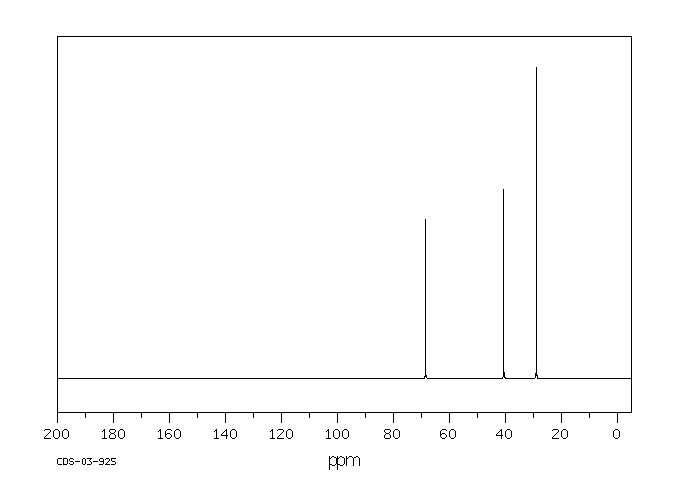
碳谱13CNMR
-
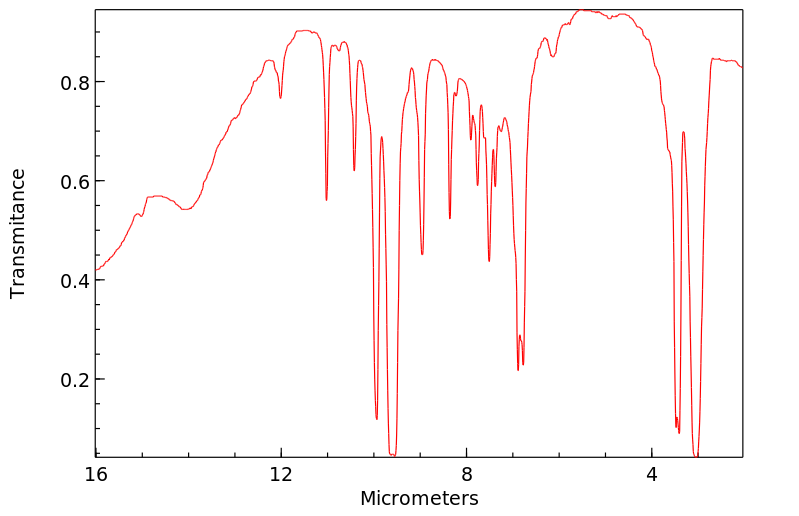
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷