1,4-dithio-erythritol | 6892-68-8
中文名称
——
中文别名
——
英文名称
1,4-dithio-erythritol
英文别名
dithioerythritol;DTE;1,4-dithiothreitol;dithiothreitol;DTT;(2R,3S)-1,4-bis(sulfanyl)butane-2,3-diol
CAS
6892-68-8
化学式
C4H10O2S2
mdl
——
分子量
154.254
InChiKey
VHJLVAABSRFDPM-ZXZARUISSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:82-84 °C(lit.)
-
沸点:247.65°C (rough estimate)
-
密度:1.156 (estimate)
-
溶解度:H2O:10 mg/mL,澄清,无色
-
最大波长(λmax):λ: 260 nm Amax: 0.400λ: 280 nm Amax: 0.050
-
稳定性/保质期:
稳定,易燃,与强氧化剂不相容。
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:42.5
-
氢给体数:4
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险等级:9
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:29309070
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P264,P270,P273,P280,P301+P312+P330,P302+P352,P305+P351+P338+P310,P332+P313,P362+P364,P501
-
危险品运输编号:3077
-
危险性描述:H302,H315,H318,H411
-
储存条件:2-8°C
SDS
| Name: | Dithioerythritol 99+% Material Safety Data Sheet |
| Synonym: | DTE; Dimercapto-2,3-Butanedio |
| CAS: | 6892-68-8 |
Synonym:DTE; Dimercapto-2,3-Butanedio
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6892-68-8 | Dithioerythritol | +99 | 229-998-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.Stench.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. No information regarding skin irritation and other potential effects was found. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Causes irritation of mucous membrane.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6892-68-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Negligible.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 82.00 - 84.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C4H10O2S2
Molecular Weight: 154.24
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures. May decompose on exposure to moist air or water.
Conditions to Avoid:
Incompatible materials, moisture.
Incompatibilities with Other Materials:
Oxidizing agents, reducing agents, alkali metals.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide, hydrogen sulfide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6892-68-8: KF2410000 LD50/LC50:
Not available.
Carcinogenicity:
Dithioerythritol - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 6892-68-8: No information available.
Canada
CAS# 6892-68-8 is listed on Canada's DSL List.
CAS# 6892-68-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6892-68-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:一种可见光诱导的硫醇和二硫化物脱硫的温和,通用,无金属方法摘要:探索了一种可见光诱导的无金属硫醇和二硫化物脱硫方法。这种自由基脱硫具有温和的条件,坚固性和出色的功能兼容性。它不仅成功地用于小分子的脱硫,而且还成功地应用于肽。DOI:10.1002/cjoc.202000607
-
作为产物:描述:参考文献:名称:用于合成二硫代赤藓醇的中间化合物及其应 用和二硫代赤藓醇的合成方法摘要:本发明公开了一种用于合成二硫代赤藓醇的中间化合物及其应用和二硫代赤藓醇的合成方法,该中间化合物为2,3‑二乙酰基硫代甲基环氧乙烷,且该中间化合物的化学式为:其可通过将2,3‑二溴甲基环氧乙烷和硫代乙酸盐进行取代反应来制备。二硫代赤藓醇的合成方法包括:将该中间化合物进行水解反应。通过2,3‑二乙酰基硫代甲基环氧乙烷可直接水解得到二硫代赤藓醇,且2,3‑二乙酰基硫代甲基环氧乙烷的制备较简单,为二硫代赤藓醇的合成提供了一种新的合成思路,并且合成路线简短,操作简便,收率较高。公开号:CN111100096B
-
作为试剂:描述:环己酚 在 sodium tetrahydroborate 、 1,4-dithio-erythritol 、 溴 、 caesium carbonate 、 sodium bromide 作用下, 以 甲醇 、 乙醇 、 乙腈 为溶剂, 反应 6.0h, 生成 ethyl 2-(2-cyclohexyl-4-mercaptophenoxy)acetate参考文献:名称:GW 501516 类似物的合成、生物学评价和分子建模摘要:制备了 GW 501516 (1) 的 11 种类似物,并在半高通量人类骨骼肌细胞试验中进行了生物学测试。分析测试表明所有类似物都引起油酸的氧化。在最有效的激动剂中,2e(2-{2-乙基-4-[(4-甲基-2-(4-三氟甲基苯基)噻唑-5-基)甲硫基]苯氧基}-2-甲基丙酸)也受到基于荧光素酶的转染试验表明该化合物是 PPARδ 的强效激动剂和 PPARα 的中度激动剂。化合物 2e 与 PPARδ 的对接表明它占据了激动剂结合位点,并表现出与 His323、His449 和 Tyr473 的关键氢键相互作用。DOI:10.1002/ardp.201000189
文献信息
-
5-Substituted-3(2H)-furanones useful for inhibition of farnesyl-protein transferase申请人:American Cyanamid Company公开号:US20030073736A1公开(公告)日:2003-04-17Compounds of Formula (I): 1 wherein R 1 , R 2 , R 3 , X, Y, Z and Q are as defined in the specification which compounds are inhibitors of Ras farnesyl-protein transferase enzyme (FPTase), and useful in treating ras oncogene-dependent tumors, such as cancers of the pancreas, colon, bladder, and thyroid and processes for the preparation of said compounds of Formula (I).
-
[EN] COMPOSITION FOR TIN-SILVER ALLOY ELECTROPLATING COMPRISING A COMPLEXING AGENT<br/>[FR] COMPOSITION POUR L'ÉLECTROGALVANISATION D'ALLIAGE D'ÉTAIN ET D'ARGENT COMPRENANT UN AGENT COMPLEXANT申请人:BASF SE公开号:WO2019185468A1公开(公告)日:2019-10-03An aqueous composition comprising (a) metal ions comprising tin ions and silver ions and (b) at least one complexing agent of formula (C1) R1-X1-S-X21[D1-X22-]nS-X3-R2, (C2) R1-X1-S-X31-D2-[X32-S-]nX3-R2, (C3) R3-X1-S-X41-[D3-X42-]nS-X3-R4 wherein X1, X3 are independently selected from a linear or branched C1-C12 alkanediyl, which may be unsubstituted or substituted by OH; X21, X22 are independently selected from X1, which may be further substituted by -X5-COOR12, -X5-SO2-O-R12, a C2 to C6 polyoxyalkylene group of formula -(O-CH2- CHR11)z-OH, or a combination thereof, and -X1-NH-CO-X6-CO-NH-X1-; X31, X32 are independently selected from a chemical bond and X1; X41, X42 are independently selected from X1; X5 is a linear or branched Ci to C10 alkyl; X6 is selected from X1 and a divalent 5 or 6 membered aromatic group; R1, R2 are independently selected from a monovalent 5 or 6 membered aromatic N- heterocyclic group comprising one N atom or two N atoms which are separated by at least one C atom, and its derivatives received by N-alkylation with a C1-C6- alkyl group, which may be substituted by --COOR12 or -SO2-O-R12, and which aromatic N- heterocyclic group may optionally further comprise, under the proviso that X21 is substituted by at least one OH, one S atom; R3, R4 are independently selected from a monovalent 5 or 6 membered aliphatic N- heterocyclic group comprising one N atom and one O atom; D1 is independently selected from S, O and NR10-; D2 is (a) a divalent 5 or 6 membered aliphatic heterocyclic ring system comprising 1 or 2 S atoms, or (b) a 5 or 6 membered aromatic heterocyclic ring system comprising at least two N atoms and optionally one or two S atoms; D3 is independently selected from S and NR10-; n is an integer of from 0 to 5; z is an integer from 1 to 50; R10 is selected from H and a linear or branched C1-C12 alkyl; R11 is selected from H and a linear or branched C1 to C6 alkyl; and R12 is selected from R10 and a cation.一种水性组合物,包括(a)包括锡离子和银离子的金属离子和(b)至少一种具有以下结构的络合剂:(C1)R1-X1-S-X21[D1-X22-]nS-X3-R2,(C2)R1-X1-S-X31-D2-[X32-S-]nX3-R2,(C3)R3-X1-S-X41-[D3-X42-]nS-X3-R4,其中X1、X3可独立地选择自线性或支链的C1-C12脂肪二亚基,可以未取代或被OH取代;X21、X22可独立地选择自X1,可以进一步被-X5-COOR12,-X5-SO2-O-R12,具有式-(O-CH2-CHR11)z-OH的C2到C6聚氧烷基,或其组合物取代,以及-X1-NH-CO-X6-CO-NH-X1-;X31、X32可独立地选择自化学键和X1;X41、X42可独立地选择自X1;X5是线性或支链的Ci到C10烷基;X6选择自X1和双价的5或6元芳香族基;R1、R2可独立地选择自包括一个N原子或两个N原子的单价5或6元芳香N-杂环基,通过N-烷基化与C1-C6烷基基团取代的衍生物,可以被--COOR12或-SO2-O-R12取代,该芳香N-杂环基可以选择性地进一步包括,在X21至少被一个OH取代的条件下,一个S原子;R3、R4可独立地选择自包括一个N原子和一个O原子的单价5或6元脂肪N-杂环基;D1可独立地选择自S、O和NR10-;D2是(a)包括1或2个S原子的双价5或6元脂肪杂环环系统,或(b)包括至少两个N原子和可选地一个或两个S原子的5或6元芳香杂环环系统;D3可独立地选择自S和NR10-;n是从0到5的整数;z是从1到50的整数;R10选择自H和线性或支链的C1-C12烷基;R11选择自H和线性或支链的C1到C6烷基;R12选择自R10和阳离子。
-
[EN] 1,2-DITHIOLANE COMPOUNDS USEFUL IN NEUROPROTECTION, AUTOIMMUNE AND CANCER DISEASES AND CONDITIONS<br/>[FR] COMPOSÉS 1,2-DITHIOLANE UTILES DANS LA NEUROPROTECTION, LES MALADIES ET LES ÉTATS AUTO-IMMUNS ET CANCÉREUX申请人:SABILA BIOSCIENCES LLC公开号:WO2018049127A1公开(公告)日:2018-03-15This invention provides confounds of the formula (I): wherein Y1, Y2 Z, X1, X2, and W are defined in the specification. These compounds are useful in the treatment of tyrosine kinases, MAPK signaling pathway kinases and Ρ13K/ΑΚΤ/mTor signaling pathway kinases-mediated diseases; or conditions, such as neurodegeneration, neuroprotection, cancer, autoimmune as well as other diseases and conditions associated with the modulation of tyrosine kinases selected from FYN, FYN Y531F, FLT3, FLT3 -ITD, BRK, ITK, FRK, BTK, BMX, SRC, FGR, YES1, LCK, HCK, RET, CSK, LYN, and ROSI; MAPK pathway kinases selected from ARAF, BRAE CRAP, ERK1 /2, MEK1, MEK2, MEK3, MEK4, MEK5. MEK6, and MEK7; and P13K/AKT/mTor pathway kinases: selected from mTor, P13K a, Ρ13Κ β, P13Kγ, and P13K δ.这项发明提供了公式(I)的混合物:其中Y1、Y2、Z、X1、X2和W在规范中定义。这些化合物在治疗酪氨酸激酶、MAPK信号通路激酶和Ρ13K/ΑΚΤ/mTor信号通路激酶介导的疾病;或病况中有用,如神经退行性疾病、神经保护、癌症、自身免疫以及与选择自FYN、FYN Y531F、FLT3、FLT3-ITD、BRK、ITK、FRK、BTK、BMX、SRC、FGR、YES1、LCK、HCK、RET、CSK、LYN和ROSI的酪氨酸激酶调节相关的其他疾病和病况;从ARAF、BRAE CRAP、ERK1/2、MEK1、MEK2、MEK3、MEK4、MEK5、MEK6和MEK7中选择的MAPK通路激酶;以及P13K/AKT/mTor通路激酶:从mTor、P13Kα、Ρ13Κβ、P13Kγ和P13Kδ中选择。
-
Molecular yardsticks. Synthesis of extended equilibrium transfer alkylating cross-link reagents and their use in the formation of macrocycles作者:Stephen J. Brocchini、Martin. Eberle、Richard G. LawtonDOI:10.1021/ja00223a061日期:1988.7Par addition de Michael synthese d'hydroxy-2' ethylthiomethyl-2 nitro-3' phenyl-1 alcadiene-2,4- ou alcatriene-2,4,6ones-1; ces composes conduisent apres oxydation a des nitro-3' benzoyl dithiacyclanes macrocycliquesPar加成德迈克尔合成d'羟基-2'乙基硫甲基-2硝基-3'苯基-1 alcadiene-2,4-ou alcatriene-2,4,6ones-1;ces 组成 conduisent apres oxydation a des nitro-3'benzoyl dithiacyclanes macrocycliques
-
Synthesis of a Stable Sulfenic Acid by Oxidation of a Thiol Bearing a Novel Bowl-type Substituent and Its Redox Reactions作者:Kei Goto、Michel Holler、Renji OkazakiDOI:10.1080/10426509708545531日期:1997.1.1A stable sulfenic acid bearing a novel bowl-type substituent was synthesized by direct oxidation of a thiol, and its structure was determined by X-ray crystallographic analysis. The redox reactions of a sulfenic acid to a thiol and to a sulfinic acid, for which only those of transient species have been reported so far, were also demonstrated conclusively.
表征谱图
-
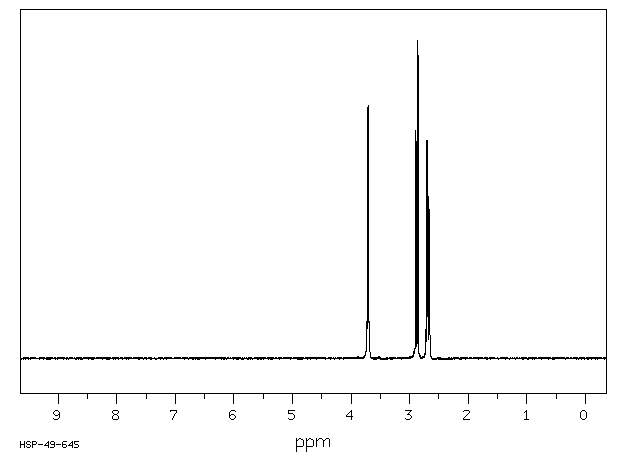
氢谱1HNMR
-
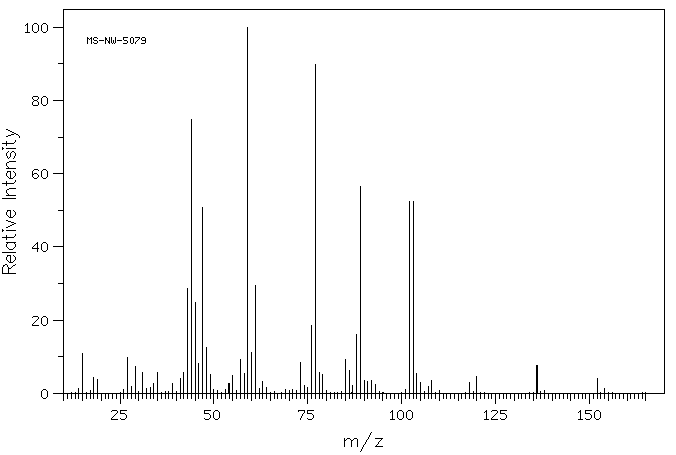
质谱MS
-
碳谱13CNMR
-
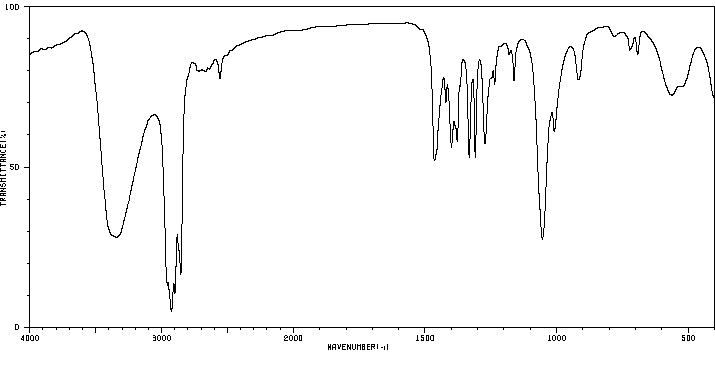
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷