萘茜 | 475-38-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:220-230 °C(lit.)
-
沸点:285.64°C (rough estimate)
-
密度:1.3366 (rough estimate)
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:14
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25,S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:NONH for all modes of transport
-
海关编码:2914690090
-
RTECS号:QL7970000
-
危险性防范说明:P501,P261,P270,P271,P264,P280,P337+P313,P305+P351+P338,P362+P364,P332+P313,P301+P312+P330,P302+P352+P312,P304+P340+P312
-
危险性描述:H302+H312+H332,H315,H319
-
储存条件:密封保存,置于通风、干燥处,并远离其他氧化物。
SDS
模块 1. 化学品
产品名称: 5,8-Dihydroxy-1,4-naphthoquinone
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
5,8-二羟基-1,4-萘醌 修改号码:2
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5,8-二羟基-1,4-萘醌
百分比: >70.0%(LC)
CAS编码: 475-38-7
俗名: 5,8-Dihydroxy-1,4-naphthalenedione , Naphthazarin
分子式: C10H6O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
5,8-二羟基-1,4-萘醌 修改号码:2
模块 7. 操作处置与储存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 深黄红色-深红色
气味: 无资料
pH: 无数据资料
熔点:
230°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: QL7970000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
5,8-二羟基-1,4-萘醌 修改号码:2
模块 12. 生态学信息
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
生物活性
Naphthazarin(DHNQ)是一种天然存在的化合物,通过多种细胞机制发挥作用,包括氧化应激、线粒体凋亡诱导因子(AIF)激活、微管解聚、干扰溶酶体功能和p53依赖性p21活化。Naphthazarin能够触发细胞凋亡,并具有抗肿瘤作用。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5,8-二甲氧基-1,4-萘二酮 5,8-dimethoxynaphthoquinone 15013-16-8 C12H10O4 218.209 —— 5,8-diacetoxy-1,4-naphthoquinone 14569-45-0 C14H10O6 274.23 —— 2,3-dibromo-5,8-dihydroxynaphthoquinone 51847-26-8 C10H4Br2O4 347.947 —— 2,3-dichloro-5,8-dihydroxy-[1,4]naphthoquinone 14918-69-5 C10H4Cl2O4 259.045 —— 5-amino-8-hydroxy-[1,4]naphthoquinone 68217-36-7 C10H7NO3 189.17 1,4-萘醌 [1,4]naphthoquinone 130-15-4 C10H6O2 158.156 —— 5,8-dihydroxy-2,3-dihydro-[1,4]naphthoquinone 4988-51-6 C10H8O4 192.171 —— 2,3,6,7-tetrabromonaphthazarin 100012-49-5 C10H2Br4O4 505.739 4-亚氨基-5-羟基-8-氨基-1(4H)-萘酮 5-amino-8-hydroxy-[1,4]naphthoquinone-1-imine 6259-68-3 C10H8N2O2 188.186 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5,7-二羟基-1,4-萘醌 5,7-dihydroxy-1,4-naphthoquinone 4923-54-0 C10H6O4 190.155 —— O-methylnaphthazarin 21418-04-2 C11H8O4 204.182 5,8-二甲氧基-1,4-萘二酮 5,8-dimethoxynaphthoquinone 15013-16-8 C12H10O4 218.209 —— naphthazarine-d2 83285-93-2 C10H6O4 192.139 —— 5,8-bis(methoxymethoxy)naphthalene-1,4-dione 220520-83-2 C14H14O6 278.262 —— 2,5,8-trihydroxy-1,4-naphthoquinone 13379-22-1 C10H6O5 206.155 2-溴-5,8-二羟基萘-1,4-二酮 2-bromo-5,8-dihydroxy-1,4-dihydronaphthalene-1,4-dione 78226-78-5 C10H5BrO4 269.051 —— 2-chloro-5,8-dihydroxy-[1,4]naphthoquinone 14918-68-4 C10H5ClO4 224.6 2-氨基-5,8-二羟基萘-1,4-二酮 2-amino-5,8-dihydroxynaphthalene-1,4-dione 87927-30-8 C10H7NO4 205.17 5-乙酰氧基-8-羟基-1,4-萘醌 5-acetoxy-8-hydroxy-1,4-naphthoquinone 14597-06-9 C12H8O5 232.193 —— 5,8-diacetoxy-1,4-naphthoquinone 14569-45-0 C14H10O6 274.23 —— 2,3-dibromo-5,8-dihydroxynaphthoquinone 51847-26-8 C10H4Br2O4 347.947 —— 5-amino-8-hydroxy-[1,4]naphthoquinone 68217-36-7 C10H7NO3 189.17 —— naphthazarin monopivalate 64278-57-5 C15H14O5 274.273 4,5,7,8-四羟基萘-1,2-二酮 4,5,7,8-tetrahydroxynaphthalene-1,2-dione 2473-16-7 C10H6O6 222.154 —— 4,5,6,8-tetrahydroxynaphthalene-1,2-dione 15257-45-1 C10H6O6 222.154 5,8-二羟基-2-甲氧基-1,4-萘醌 5,8-dihydroxy-2-methoxy-[1,4]naphthoquinone 14918-66-2 C11H8O5 220.182 —— 5-benzoyloxy-8-hydroxynaphthalene-1,4-dione 192801-82-4 C17H10O5 294.263 —— 2-(N,N-dimethylamino)naphthazarin 117554-13-9 C12H11NO4 233.224 —— 2-propylamino-5,8-dihydroxy-1,4-naphthoquinone 101663-68-7 C13H13NO4 247.251 —— 5,6-diamino-8-hydroxy-[1,4]naphthoquinone —— C10H8N2O3 204.185 2-(丁基氨基)-5,8-二羟基萘-1,4-二酮 2-n-butylamino-5,8-dihydroxy-1,4-naphthoquinone 71541-74-7 C14H15NO4 261.277 —— (5,8-Dihydroxy-1,4-dioxo-1,4-dihydro-naphthalen-2-ylsulfanyl)-acetic acid 65726-71-8 C12H8O6S 280.258 —— 2-n-hexylamino-5,8-dihydroxy-1,4-naphthoquinone —— C16H19NO4 289.331 —— 5,8-dihydroxy-2,3-dihydro-[1,4]naphthoquinone 4988-51-6 C10H8O4 192.171 —— S-(5,8-dihydroxy-1,4-naphthoquinon-2-yl)-2-mercaptopropionic 65726-90-1 C13H10O6S 294.285 —— 4-(5,8-Dihydroxy-1,4-dioxo-1,4-dihydro-naphthalen-2-ylsulfanyl)-butyric acid 65758-35-2 C14H12O6S 308.312 —— 2-phenylmethylamino-5,8-dihydroxy-1,4-naphthoquinone 101663-65-4 C17H13NO4 295.295 —— 5,8-dihydroxy-3-methyl-1,2,3,4-tetrahydro-9,10-anthraquinone 14978-56-4 C15H14O4 258.274 —— S-(5,8-dihydroxy-1,4-naphthoquinon-2-yl)-2-mercaptopropionic acid —— C13H10O6S 294.285 —— 2-phenylamino-5,8-dihydroxy-1,4-naphthoquinone 47123-72-8 C16H11NO4 281.268 —— 8-hydroxy-5-p-tosyloxy-1,4-naphthoquinone 288571-73-3 C17H12O6S 344.345 —— 5,8-dihydroxy-2-(pyrrolidin-1-yl)naphthalene-1,4-dione 129961-63-3 C14H13NO4 259.262 —— 2,3,6,7-tetrabromonaphthazarin 100012-49-5 C10H2Br4O4 505.739 —— N-(5,8-dihydroxy-1,4-naphthoquinone-2-yl)piperidine 129961-64-4 C15H15NO4 273.288 —— 5,8-dihydroxy-3-methyl-1,2-dihydro-9,10-anthraquinone 137788-35-3 C15H12O4 256.258 —— 6-demethoxy-1-deoxyaustrocortirubin 137788-44-4 C15H14O5 274.273 —— 5,8-dihydroxy-2-(4-phenylpiperazin-1-yl)naphthalene-1,4-dione 943867-72-9 C20H18N2O4 350.374 —— 2,5,8-Triacetoxy-[1,4]naphthochinon 78226-66-1 C16H12O8 332.266 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:521.芳族酮-烯醇。第二部分 一些新的2:3-dihydro-1:4-萘醌和-蒽醌摘要:DOI:10.1039/jr9520002759
-
作为产物:描述:2,3,6,7-tetrabromonaphthazarin 在 铁粉 、 氧气 作用下, 以 溶剂黄146 、 sodium hydroxide 为溶剂, 反应 0.67h, 以83%的产率得到萘茜参考文献:名称:卤代萘甲素和醌茜素的还原脱卤是制备母体化合物的简单途径摘要:摘要 通过 2-氯-3-烷基-、2,3-二氯(二溴)-、三氯-(三溴)-和四氯(四溴)-萘并和 2,3-二氯喹吖啶与 Fe/HOAc 反应,然后氧化在温和碱性条件下的空气中,以良好至极好的收率获得了相应的萘并芴素。DOI:10.1080/00397919808007029
文献信息
-
Synthesis and biological evaluation of novel (l)-α-amino acid methyl ester, heteroalkyl, and aryl substituted 1,4-naphthoquinone derivatives as antifungal and antibacterial agents作者:Vishnu K. Tandon、Dharmendra B. Yadav、Ravindra V. Singh、Ashok K. Chaturvedi、Praveen K. ShuklaDOI:10.1016/j.bmcl.2005.08.032日期:2005.12A series of (S)-N-(1,4-naphthoquinon-2-yl)-alpha-amino acid methyl esters 3-9, 2-N,N-dialkylamino-1,4-naphthoquinones 10-11 and 2-hydroxy-3-(2'-mercaptoimidazolyl)-1,4-naphthoquinones and their cyclic analogs 12-15 were synthesized and evaluated for antifungal and antibacterial activities. The structure-activity relationships of these compounds were studied and the results show that the compounds 9b
-
(-)-Shikimic Acid as a Chiral Building Block for the Synthesis of New Cytotoxic 6-Aza-Analogues of Angucyclinones作者:Natalia Quiñones、Santiago Hernández、Luis Espinoza Catalán、Joan Villena、Ivan Brito、Alan Cabrera、Cristian Salas、Mauricio CuellarDOI:10.3390/molecules23061422日期:——The cytotoxic activity of the new synthesized compounds and five angucyclinones, previously reported, was evaluated in vitro against three cancer cell lines: PC-3 (prostate cancer), HT-29 (colon cancer), MCF-7 (breast cancer), and one non-tumoral cell line, human colon epithelial cells (CCD841 CoN). Our results showed that most 6-azadiene derivatives exhibited significant cytotoxic activities, which我们描述了九种新的 angucyclinone 6-aza-analogues 的合成,通过莽草酸衍生物-氮杂二烯 13 与不同萘醌之间的异狄尔斯-阿尔德反应实现。先前报道的新合成化合物和五种环环素酮的细胞毒活性在体外针对三种癌细胞系进行了评估:PC-3(前列腺癌)、HT-29(结肠癌)、MCF-7(乳腺癌)和一种非肿瘤细胞系,人结肠上皮细胞 (CCD841 CoN)。我们的结果表明,大多数 6-氮杂二烯衍生物表现出显着的细胞毒活性,其 IC50 值(小于 10 μM)证明了这一点,尤其是对于最敏感的细胞 PC-3 和 HT-29。从化学的角度来看,取决于A环的保护基团和D环上的取代模式,就其效力和选择性而言,细胞毒性引发了这些化合物。因此,根据这些化学特征,对于每种癌细胞系,最有希望的药物是 PC-3 细胞的 7a、17 和 19c;HT-29 细胞为 7a、17 和 20,MCF-7 细胞为
-
‘On water’: unprecedented nucleophilic substitution and addition reactions with 1,4-quinones in aqueous suspension作者:Vishnu K. Tandon、Hardesh K. MauryaDOI:10.1016/j.tetlet.2009.07.149日期:2009.10Unique nucleophilic substitution and addition reactions of 1,4-quinones in aqueous suspension with aromatic amines, primary aliphatic amines, amino acid, ester of amino acid, heterocyclic amines, hydrazine, amide, and thioethers are described in absence of catalyst against the traditional synthetic routes of these reactions in non-aqueous medium in presence of catalyst.
-
Rhenium-based molecular rectangular boxes with large inner cavity and high shape selectivity towards benzene molecule作者:Rong-Tang Liao、Woei-Chyuan Yang、P. Thanasekaran、Chen-Chuan Tsai、M. Sathiyendiran、Yen-Hsiang Liu、T. Rajendran、Hsiu-Mei Lin、Tien-Wen Tseng、Kuang-Lieh LuDOI:10.1039/b802777c日期:——The self-assembly of rhenium-based rectangular boxes with a large inner cavity can be achieved via a simple one-step synthetic route; these molecules selectively recognize planar aromatic molecules, benzene in particular.通过简单的一步合成方法,可以实现基于铼的矩形盒子的自组装,这些分子具有较大的内部空腔;它们能够选择性地识别平面芳香分子,尤其是苯。
-
Syntheses of Angucyclinones Related to Ochromycinone作者:Tomas Rozek、Wit Janowski、John M. Hevko、Edward R. T. Tiekink、Suresh Dua、David J. M. Stone、John H. BowieDOI:10.1071/c97151日期:——
Two synthetic approaches have been investigated for the syntheses of model angucyclinones related to ochromycinone. The first involves a Diels–Alder/Friedel–Crafts strategy in which the Diels–Alder adduct formed between dimethyl acetylenedicarboxylate and 3-ethenyl-5,5-dimethylcyclohex-2-en-1- one was converted into 6,6-dimethyl-8-oxo-5,6,7,8-tetrahydronaphthalene-1,2-dicarboxylic anhydride, which was then reacted with benzene in a Friedel–Crafts reaction. Acid-catalysed cyclization of the Friedel–Crafts products gave 3,3-dimethyl-3,4-dihydrobenz[a]anthracene-1,7,12(2H)-trione (3) in poor yield. Angucyclinones related to (3) are formed (in 40–50% overall yield) by aromatization of the adduct formed between the appropriate 1,4-naphthoquinone and 3-[(E)-2-methoxyethenyl]- 5,5-dimethylcyclohex-2-en-1-one (this dienone reacts with itself by a Diels–Alder process to yield an adduct which decomposes to 3,3,8,8-tetramethyl-3,4,7,8-tetrahydroanthracene-1,6(2H,5H)-dione). When the substituted 1,4-naphthoquinone is unsymmetrical, a boron triacetate assisted Diels–Alder reaction gives a single regioisomer (e.g. X-ray investigations indicate that 8-hydroxy-3,3-dimethyl-3,4-dihydrobenz[a]anthracene-1,7,12(2H)-trione is the product from 5-hydroxy-1,4-naphthoquinone). An X-ray structural study of the Diels–Alder adduct in the above reaction confirms the operation of an endo cyclization.
研究了两种合成方法,以合成模型 angucyclinones。第一种是 Diels-Alder/Friedel-Crafts 策略,其中 乙炔二羧酸二甲酯与 3-乙烯基-5,5-二甲基-2-丙酮之间形成的 Diels-Alder 加合物。 3-乙烯基-5,5-二甲基环己-2-烯-1-酮之间形成的 Diels-Alder 加合物转化为 6,6-二甲基-8-氧代-5,6,7,8-四氢萘-1,2-二羧酸酐、 然后与苯发生弗里德尔-卡夫斯反应。 在酸催化下,Friedel-Crafts 反应产物环化生成了 3,3-二甲基-3,4-二氢苯并[a]蒽-1,7,12(2H)-三酮(3) (3) ,收率较低。与(3)相关的 Angucyclinones 由芳香族化合物生成(总收率为 40-50%)。 的加合物发生芳香化反应而形成(总收率为 40-50%)。 之间的加合物发生芳香化反应,生成与 (3) 相关的 Angucyclinones(总收率为 40-50%)。 3-[(E)-2-methoxyethenyl]- 5,5-二甲基环己-2-烯-1-酮之间形成的加合物进行芳香化反应(这种二烯酮通过 Diels-Alder 反应生成加合物,加合物分解为 3,3,8,8-四甲基-3,4,7,8-四氢蒽-1,6(2H,5H)-二酮)。 当取代的 1,4-萘醌不对称时,三乙酸硼 三乙酸硼辅助的 Diels-Alder 反应会产生单一的区域异构体(例如,X 射线 研究表明 8-羟基-3,3-二甲基-3,4-二氢苯并[a]蒽-1,7,12(2H)-三酮 是 5-羟基-1,4-萘醌的产物)。X 射线结构研究 对上述反应中的 Diels-Alder 加合物进行的 X 射线结构研究证实了 内环化反应。
表征谱图
-
氢谱1HNMR
-
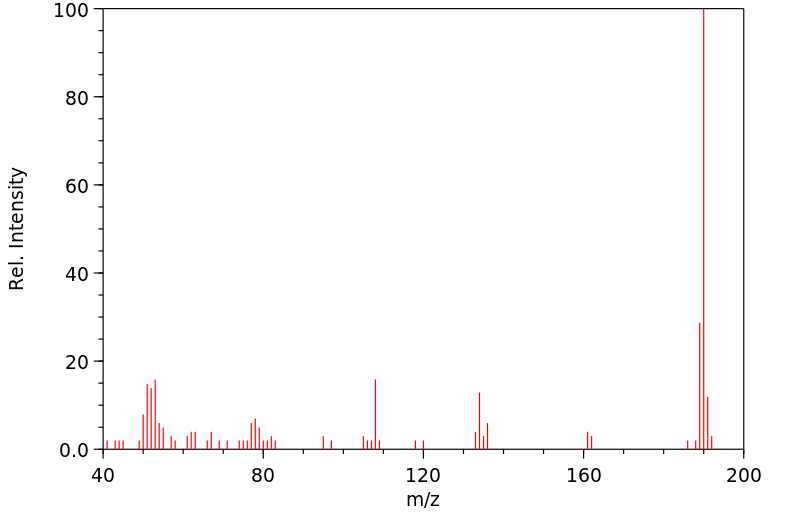
质谱MS
-
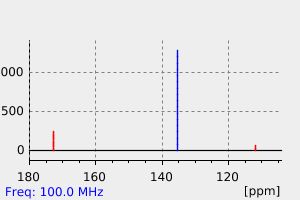
碳谱13CNMR
-
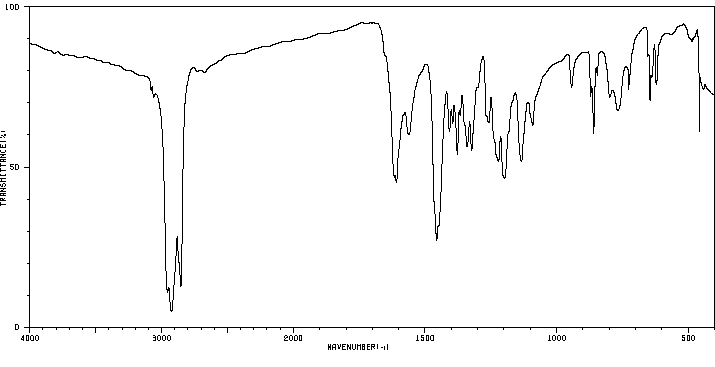
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息