2,5,8-trihydroxy-1,4-naphthoquinone | 13379-22-1
中文名称
——
中文别名
——
英文名称
2,5,8-trihydroxy-1,4-naphthoquinone
英文别名
2,5,8-trihydroxynaphthalene-1,4-dione;2-hydroxynaphthazarine;2-hydroxynaphthazarin;naphthopurpurin;2,5,8-trihydroxy-[1,4]naphthoquinone;2,5,8-Trihydroxy-[1,4]naphthochinon
CAS
13379-22-1
化学式
C10H6O5
mdl
——
分子量
206.155
InChiKey
ZFXYVCPRQYVJDR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.92
-
重原子数:15.0
-
可旋转键数:0.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:94.83
-
氢给体数:3.0
-
氢受体数:5.0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5,8-二羟基-2-甲氧基-1,4-萘醌 5,8-dihydroxy-2-methoxy-[1,4]naphthoquinone 14918-66-2 C11H8O5 220.182 萘茜 5,8-Dihydroxy-1,4-naphthoquinone 475-38-7 C10H6O4 190.155 —— 2,5,8-Triacetoxy-[1,4]naphthochinon 78226-66-1 C16H12O8 332.266 2-溴-5,8-二羟基萘-1,4-二酮 2-bromo-5,8-dihydroxy-1,4-dihydronaphthalene-1,4-dione 78226-78-5 C10H5BrO4 269.051 —— 2-chloro-5,8-dihydroxy-[1,4]naphthoquinone 14918-68-4 C10H5ClO4 224.6 —— 2,3-dichloro-5,8-dihydroxy-[1,4]naphthoquinone 14918-69-5 C10H4Cl2O4 259.045 —— 5,8-dihydroxy-2,3-dihydro-[1,4]naphthoquinone 4988-51-6 C10H8O4 192.171 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5,8-二羟基-2-甲氧基-1,4-萘醌 5,8-dihydroxy-2-methoxy-[1,4]naphthoquinone 14918-66-2 C11H8O5 220.182 —— 2-butoxy-5,8-dihydroxynaphthalene-1,4-dione 1599483-53-0 C14H14O5 262.262 —— 5-hydroxy-2,8-dimethoxy-1,4-naphthoquinone 83571-05-5 C12H10O5 234.208 —— 2-Acetoxy-naphthazarin 15012-54-1 C12H8O6 248.192 —— 2,5,8-trimethoxynaphthalene-1,4-dione 62385-85-7 C13H12O5 248.235 —— 2-bromo-3,5,8-trihydroxynaphthalene-1,4-dione 1311459-29-6 C10H5BrO5 285.051 —— 5,8-dihydroxy-2-[(trifluoromethanesulfonyl)oxy]-1,4-naphthoquinone 191228-91-8 C11H5F3O7S 338.218 —— 2,2′-methylenebis(3,5,8-trihydroxynaphthoquinone) —— C21H12O10 424.32 —— 2-chloro-5,8-dihydroxy-[1,4]naphthoquinone 14918-68-4 C10H5ClO4 224.6 —— 5,7-dihydroxy-8-methoxy-1,4-naphthoquinone 191228-93-0 C11H8O5 220.182 —— 5,6-dimethoxy-8-hydroxy-1,4-naphthoquinone 83571-07-7 C12H10O5 234.208 —— 5,6,8-trimethoxynaphthalene-1,4-dione 83571-08-8 C13H12O5 248.235 —— 5-amino-6,8-dihydroxynaphthalene-1,4-dione 1184950-93-3 C10H7NO4 205.17 紫红孢菌素 bostrycin 21879-81-2 C16H16O8 336.298 —— 6,9-dihydroxy-2-methyl-2-(4-methyl-3-pentenyl)-2H-naphtho<2,3-b>pyran-5,10-dione 173167-16-3 C20H20O5 340.376 - 1
- 2
反应信息
-
作为反应物:描述:2,5,8-trihydroxy-1,4-naphthoquinone 在 四乙酰基二硼氧烷 、 potassium hydrogencarbonate 作用下, 以 乙腈 、 苯 为溶剂, 反应 25.0h, 生成 1,4-二羟基-2-甲氧基-7-甲基蒽-9,10-二酮参考文献:名称:Burns, Christopher J.; Gill, Melvyn, Australian Journal of Chemistry, 1991, vol. 44, # 10, p. 1447 - 1458摘要:DOI:
-
作为产物:描述:参考文献:名称:Bekaert, Alain; Andrieux, Jean; Plat, Michel, Bulletin de la Societe Chimique de France, 1986, # 2, p. 314 - 316摘要:DOI:
文献信息
-
Chemistry of naphthazarin derivatives 13. Conformational analysis of 3-(alk-1-enyl)-2-hydroxy-1,4-naphthoquinones by quantum chemistry methods作者:V. P. Glazunov、D. V. Berdyshev、A. Ya. Yakubovskaya、N. D. PokhiloDOI:10.1007/s11172-006-0480-z日期:2006.10The molecular structures of various conformers of 2-hydroxy-1,4-naphthoquinone; 3-(alk-1-enyl)-2-hydroxy-1,4-naphthoquinones; 2,5,8-trihydroxy-1,4-naphthoquinone; and 3-(alk-1-enyl)-2,5,8-trihydroxy-1,4-naphthoquinones were studied by density functional theory (B3LYP/6-31(d), B3LYP/6-31(d, p)) and ab initio (MP2/6-31G, MP2/6-31(d)) methods. The strengths of the intramolecular hydrogen bonds formed by the β-hydroxy group with the O atom at C(1) and with the double bond π-electrons of the alkenyl substituents in the quinonoid rings were estimated. The compounds studied mainly exist as rotamers with the former-type hydrogen bonds. The splitting of the quinonoid bands of the stretching vibrations of the β-hydroxy group in the IR spectra of 3-(alk-1-enyl)-2-hydroxy-1,4-naphthoquinones and 3-(alk-1-enyl)-2,5,8-trihydroxy-1,4-naphthoquinones in hexane solutions is due to the existence of rotamers formed upon internal rotation of the alkenyl substituent.通过密度泛函理论 (B3LYP/6-31(d), B3LYP/6-31(d, p)) 和从头算 (MP2/6-31G, MP2/6-31(d)) 方法研究了2-羟基-1,4-萘醌、3-(链烯-1-基)-2-羟基-1,4-萘醌、2,5,8-三羟基-1,4-萘醌和3-(链烯-1-基)-2,5,8-三羟基-1,4-萘醌的各种构象分子的分子结构。估算了β-羟基与C(1)位氧原子及与醌环上链烯基取代基双键π电子形成的分子内氢键的强度。所研究的化合物主要以形成前一种类型氢键的旋转异构体形式存在。3-(链烯-1-基)-2-羟基-1,4-萘醌和3-(链烯-1-基)-2,5,8-三羟基-1,4-萘醌在己烷溶液中的红外光谱中β-羟基伸缩振动的醌带分裂是由于链烯基取代基内旋转形成的旋转异构体存在所致。
-
[EN] NAPTHOQUINONES, PRO-DRUGS, AND METHODS OF USE THEREOF<br/>[FR] NAPHTOQUINONES, PROMÉDICAMENTS, ET LEURS PROCÉDÉS D'UTILISATION申请人:UNIV TEXAS公开号:WO2017106624A1公开(公告)日:2017-06-22Provided herein are naphthoquinones compounds such as those with a hydrogen bond donating group of the formula (I): wherein: R1, R2, R3, R4, R5, and n are as defined herein. Also provided herein are pharmaceutical composition of the present compounds and methods of treatment using the compounds including their use in the treatment of cancer.提供在本说明书中的萘醌化合物,例如具有式(I)中的氢键供体基团的那些:其中:R1、R2、R3、R4、R5和n如本文所述定义。此外,还提供了本化合物的药物组合物以及使用这些化合物进行治疗的方法,包括它们在癌症治疗中的应用。
-
Electronic spectra of substituted naphthoquinones作者:I. Singh、R.T. Ogata、R.E. Moore、C.W.J. Chang、P.J. ScheuerDOI:10.1016/s0040-4020(01)90989-5日期:1968.1aid to structural elucidation of these compounds the electronic spectra of a large number of substituted naphthoquinones were examined. The bands in the 240–600 mμ region of the electronic spectra of 1,4-naphthoquinone, juglone, and naphthazarin have been assigned to either benzenoid or quinoid electronic excitations and the effect of substitution on the position of these bands has been systematically
-
1,4-Conjugate addition of 2-hydroxynaphthazarins to acyclic and cyclic α,β-unsaturated ketones. Prototropic ring-chain tautomerism of the adducts作者:N. N. Balaneva、O. S. Radchenko、V. P. Glazunov、V. L. NovikovDOI:10.1007/s11172-012-0264-6日期:2012.10TsOH-Catalyzed reactions of 6,7-disubstituted 2-hydroxynaphthazarins and acyclic or cyclic α,β-enones afforded the corresponding Michael-type adducts, viz., 3-substituted naphthazarins in 17–78% yields. 1H and 13C NMR and IR spectroscopy showed that the adducts with cyclohexenone and methyl vinyl ketone exist in CDCl3 solutions as mixtures of open-chain and cyclic tautomers, whereas the adducts with
-
The Reductive Dehalogenation of Halo-Substituted Naphthazarins and Quinizarins as a Simple Route to Parent Compounds作者:Victor Ph. Anufriev、Galina V. Malinovskaya、Vyacheslav L. Novikov、Nadezhda N. Balanyova、Sergey G. PolonikDOI:10.1080/00397919808007029日期:1998.6Abstract By the reaction of 2-chloro-3-alkyl-, 2,3-dichloro(dibromo)-, trichloro-(tribromo)-and tetrachloro(tetrabromo)-naphthazarins and 2,3-dichloroquinizarin with Fe/HOAc followed by oxidation with an air in a mild basic conditions the corresponding naphthazarins and quinizarin were obtained in good to excellent yields.
表征谱图
-
氢谱1HNMR
-
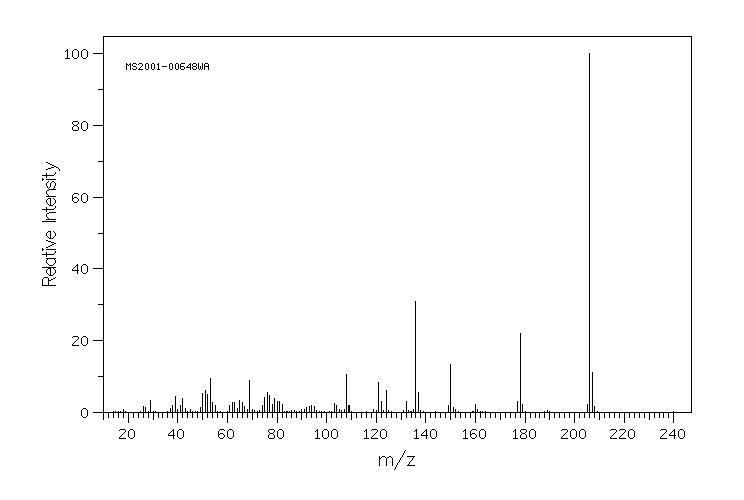
质谱MS
-
碳谱13CNMR
-
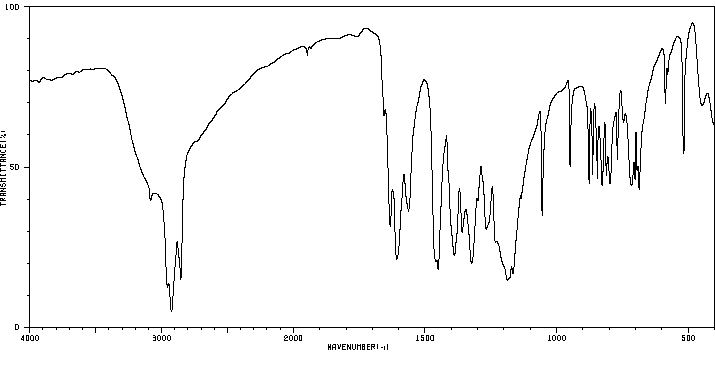
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮