2-羟基-1-(4-羟基-3-甲氧基苯基)乙酮 | 18256-48-9
中文名称
2-羟基-1-(4-羟基-3-甲氧基苯基)乙酮
中文别名
——
英文名称
2,4'-dihydroxy-3'-methoxyacetophenone
英文别名
2-hydroxy-1-(4-hydroxy-3-methoxyphenyl)ethanone;α-hydroxyacetovanillone
CAS
18256-48-9
化学式
C9H10O4
mdl
——
分子量
182.176
InChiKey
QNMANLUEFQNQCX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160–161°C
-
沸点:370.4±27.0 °C(Predicted)
-
密度:1.292±0.06 g/cm3(Predicted)
-
LogP:0.558 (est)
计算性质
-
辛醇/水分配系数(LogP):-0.1
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:66.8
-
氢给体数:2
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 香草乙酮 1-(3-methoxy-4-hydroxyphenyl)ethanone 498-02-2 C9H10O3 166.177 2-溴-1-(4-羟基-3-甲氧基苯基)乙酮 2-bromo-1-(4-hydroxy-3-methoxyphenyl)ethanone 69638-06-8 C9H9BrO3 245.073 —— 4-Amino-butyric acid 2-(4-hydroxy-3-methoxy-phenyl)-2-oxo-ethyl ester 284043-11-4 C13H17NO5 267.282 香草醛 vanillin 121-33-5 C8H8O3 152.15 香草基扁桃酸乙酯 ethyl 4-hydroxy-3-methoxymandelate 52058-11-4 C11H14O5 226.229 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(3,4-dimethoxyphenyl)-2-hydroxyethan-1-one 37803-48-8 C10H12O4 196.203 —— 2-acetoxy-1-(4-hydroxy-3-methoxy-phenyl)-ethanone 139473-80-6 C11H12O5 224.213
反应信息
-
作为反应物:参考文献:名称:«物质Z»。关于肾上腺皮质成分及相关物质第100部分摘要:«替代的隔离。描述了来自肾上腺提取物的Z»。发现该物质与4,ω-二羟基-3-甲氧基-苯乙酮相同,后者以纯结晶形式合成用于比较。表格已准备好。描述了位置异构体3,ω-二羟基-4-甲氧基-苯乙酮(“ Iso-Iso-Z”)的合成。DOI:10.1002/hlca.19590420610
-
作为产物:参考文献:名称:异槲皮酚的合成及其抗炎活性。摘要:我们在这里报告了异方苯酚的合成,异方苯酚是枫糖浆中发现的多酚化合物quebecol的前所未有的结构异构体。制备异白酚的方法学涉及关键步骤,即由α-酮酸酯前体形成二溴代烯烃,然后进行Suzuki-Miyaura双反应。通过监测其抑制LPS诱导的IL-6分泌的能力,研究了异黄柏对巨噬细胞的抗炎活性。结果表明,这种新化合物具有比其天然异构体更高的生物活性。这项研究中还制备了quebecol,异quebecol和模型类似物2,3,3-三苯丙醇的前体和衍生物。DOI:10.1016/j.bmc.2017.01.050
文献信息
-
New Phototriggers:<sup>1</sup> Extending the <i>p</i>-Hydroxyphenacyl π−π* Absorption Range作者:Peter G. Conrad、Richard S. Givens、Jörg F. W. Weber、Karl KandlerDOI:10.1021/ol005856n日期:2000.6.1[equation--see text] Introducing 3-methoxy or 3,5-dimethoxy substituents on the 4-hydroxyphenacyl (pHP) photoremovable protecting group has been explored with two excitatory gamma-amino acids, L-glutamic acid and gamma-amino butyric acid (GABA). These substituents significantly extend the absorption range of the pHP chromophore, e.g., the tail of absorption bands of 2a,b extend above 400 nm, well beyond
-
Enzyme catalyzed hydroxymethylation of aromatic aldehydes with formaldehyde. Synthesis of hydroxyacetophenones and (S)-benzoins作者:Ayhan S Demir、Peruze Ayhan、A.Cigdem Igdir、A.Nese DuyguDOI:10.1016/j.tet.2004.06.015日期:2004.7Benzaldehyde lyase from the Pseudomonas Fluorescens catalyzed reaction of aromatic aldehydes with formaldehyde providing 2-hydroxy-1-arylethan-1-one in high yields via an acyloin linkage. Kinetic resolution of rac-benzoins with formaldehyde providing (S)-benzoins and 2-hydroxy-1-arylethan-1-one via C–C bond cleavage and a bond formation reaction.
-
Rapid flow-through fractionation of biomass to preserve labile aryl ether bonds in native lignin作者:Hao Zhou、Jia Yun Xu、Yingjuan Fu、Haiguang Zhang、Zaiwu Yuan、Menghua Qin、Zhaojiang WangDOI:10.1039/c9gc02315a日期:——dissolved lignin from the reactor in time and space, thereby preserving these labile aryl ether bonds in native lignin. The application of RFF of poplar wood with a short residence time of 2.6 min attained 75% delignification with an equivalent of the β-O-4 motif in native lignin. Structure-preserved lignins (β-O-4 retention, 75.0%–85.4%) were also harvested from wheat straw with good lignin yields (61.7%–78木质素是维管束植物的第二大成分,是地球上最丰富的可再生芳香族聚合物。木质素增值的吸引力在于它可以通过分馏和提质转化为高价值的芳香族化学品和生物燃料。文献已经证明,天然木质素中芳基醚键的存在是转化的关键因素,而碳水化合物优先生产工艺(例如纸浆和纤维素乙醇生产)中的常规工业木质素经过密集的冷凝,并且由于这些键而缺乏这些联系。激烈的脱木素条件。在这里,通过使用β-O-4木质素模型二聚体GG,我们揭示了GG的急剧降解以及相对稳定的中间β-O-4二聚体C 6 C的同步形成。3在72wt%的甲酸水溶液和130°C的条件下的前5分钟内,烯醇醚和甲酰化的烯醇醚适合于生物质分馏。基于这些发现,我们提出了一种简单但有效的快速流通分级分离(RFF)策略,该策略可在时间和空间上从反应器中分离出溶解的木质素,从而在天然木质素中保留这些不稳定的芳基醚键。杨木的RFF短停留时间2.6分钟的应用获得了75%的脱木质素作用
-
METHOD FOR THE BREAKDOWN OF LIGNIN申请人:Voitl Tobias公开号:US20100121110A1公开(公告)日:2010-05-13The invention describes a method for the direct production of molecules with a minimum molecular weight of 78 g/mol by the breakdown of lignin, lignin derivatives, lignin fragments, and/or lignin-containing substances or mixtures in the presence of at least one polyoxometallate and preferably in the presence of a radical scavenger in a liquid medium.
-
Formation of hydroxycinnamoylamides and α-hydroxyacetovanillone in cell cultures of Solanum khasianum作者:Ute Mühlenbeck、Albertus Kortenbusch、Wolfgang BarzDOI:10.1016/0031-9422(96)00173-2日期:1996.8Abstract In elicitor-treated photomixotrophic Solanum khasianum cell cultures, nine phenolic compounds accumulated in the cell culture medium. They were isolated and structurally identified as the cis - and trans -isomers of N - p -coumaroyloctopamine, N -feruloyloctopamine, N - p -coumaroyltyramine and N -feruloyltyramine as well as α-hydroxyacetovanillone. The cis -isomers of the hydroxycinnamoyl moieties
表征谱图
-
氢谱1HNMR
-
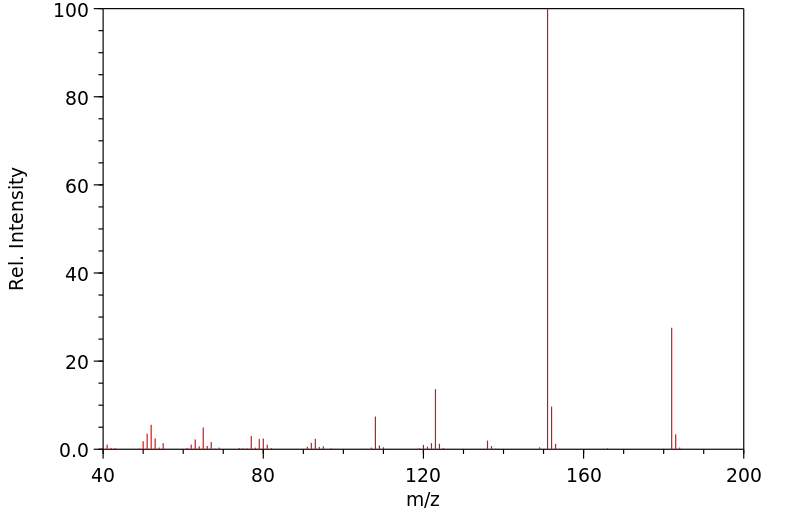
质谱MS
-
碳谱13CNMR
-
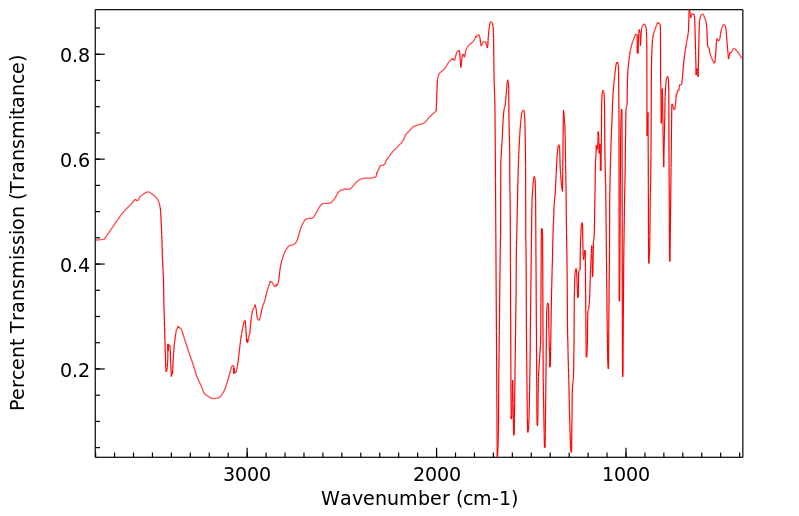
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷