丁烷 | 106-97-8
中文名称
丁烷
中文别名
正丁烷;甲基乙基甲烷
英文名称
Butane
英文别名
n-butane;HC-600
CAS
106-97-8
化学式
C4H10
mdl
MFCD00009424
分子量
58.1234
InChiKey
IJDNQMDRQITEOD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−138 °C(lit.)
-
沸点:−0.5 °C(lit.)
-
密度:0.579 g/mL at 20 °C(lit.)
-
蒸气密度:2.11 (vs air)
-
闪点:45
-
暴露限值:TLV-TWA 800 ppm (~1920 mg/m3) (ACGIH), 500 ppm (1200 mg/m3) (MSHA).
-
介电常数:1.4(-1℃)
-
LogP:2.890
-
物理描述:Colorless gas with a gasoline-like or natural gas odor. [Note: Shipped as a liquefied compressed gas. A liquid below 31°F.]
-
颜色/状态:Colorless gas [Note: Shipped as a liquefied compressed gas. A liquid below 31 degrees F]
-
气味:Faint, disagreeable odor
-
溶解度:In water, 61.2 mg/L at 25 °C
-
蒸汽密度:2.046 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1820 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 0.95 atm-cu m/mole at 25 °C (est)
-
大气OH速率常数:2.54e-12 cm3/molecule*sec
-
自燃温度:550 °F (287 °C)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
粘度:7.5 at 300 K; 9.9 at 400 K; 12.2 at 500 K; 14.5 at 600 K (all in uPa.s) (gas)
-
腐蚀性:Has no corrosive action on metals
-
燃烧热:-19,512 BTU/lb= -10,840 cal/g= -453.85x10+5 J/kg
-
汽化热:22.39 kJ/mol at normal BP
-
表面张力:14.7 dynes/cm at 0 °C
-
电离电位:10.63 eV
-
气味阈值:Odor Threshold Low: 1.2 [ppm]; Odor Threshold High: 6.5 [ppm]; Odor threshold from ACGIH
-
折光率:Index of refraction: 1.3326 at 20 °C/D
-
保留指数:400
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强酸、强碱、卤素。
-
聚合危害:不发生聚合。
-
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:4
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
进行了一项研究,以确定挥发性烃类化合物,如丙烷、正丁烷和异丁烷,是否在小鼠体内被代谢。在吸入这些气体的小鼠中,从丙烷中产生了异丙醇和丙酮,从正丁烷中产生了sec-丁醇和甲基乙基甲酮,从异丁烷中产生了tert-丁醇作为相应的代谢物。此外,发现肝微粒体含有参与这些代谢的酶系统。体外反应表明,肝微粒体可以将丙烷转化为异丙醇,将正丁烷转化为sec-丁醇,将异丁烷转化为tert-丁醇。据推测,烃类化合物首先被微粒体酶系统转化为(omega-1)-醇,然后通过醇脱氢酶转化为相应的酮。
A study was conducted to establish whether volatile hydrocarbons, such as propane, n-butane and iso-butane, are metabolized in mice or not. In mice having inhaled these gases, isopropanol and acetone were yielded from propane, sec-butanol and methyl ethyl ketone from n-butane, and tert-butanol from iso-butane as the respective metabolites. In addition, liver microsomes were found to contain the enzymic system participating in these metabolisms. In vitro reactions with liver microsomes produced isopropanol from propane, sec-butanol from n-butane, and tert-butanol from iso-butane. It was assumed that hydrocarbons were first converted to (omega-1)-alcohols by microsomal enzyme system and then to corresponding ketones by alcohol dehydrogenase.
来源:Hazardous Substances Data Bank (HSDB)
代谢
微粒体酶系统能够将丁烷氧化成其母醇。
Microsomal enzyme systems have been found that oxidize butane to its parent alcohol.
来源:Hazardous Substances Data Bank (HSDB)
代谢
丁烷的羟基化在大鼠肝微粒体中发生,主要代谢产物是2-丁醇。正丁烷是已证明能够与细胞色素P450结合的最低分子量烷烃。如果在哺乳动物中形成的主要代谢物是2-丁醇,那么它预期会通过呼出的空气排出。2-丁醇还可能与葡萄糖醛酸结合,或者被氧化成甲基乙基酮,后者也会被呼出。
Hydroxylation of butane ... /occurs/ in rat liver microsomes to yield 2-butanol as the major metabolite. n-Butane is the lowest molecular weight alkane ... demonstrated to substrate-bind with cytochrome p450. ... If 2-butanol is the major metabolite formed in mammals, it would be expected to be eliminated in expired air. ... 2-Butanol may also be conjugated with glucuronic acid or be oxidized to methyl ethyl ketone which in turn is expired.
来源:Hazardous Substances Data Bank (HSDB)
代谢
纯净的液化石油气(LPG),是由丁烷、异丁烷和丙烷混合而成的,通常通过吸入方式滥用。关于这些物质在哺乳动物体内的代谢情况,人们知之甚少。其他一些碳氢化合物,包括正己烷和环己烷的代谢,已经在体外使用各种肝脏制剂进行了研究,并通过静态顶空技术分析了代谢物...从环己烷中形成了环己醇和环己酮,正如预测的那样,从正己烷中形成了1-、2-和3-己醇以及2-己酮。除了丙烯和异丁烷之外,研究的其他化合物发现了二级醇,以及从丙烷和正丁烷分别形成的2-丙酮和2-丁酮。三名因滥用LPG而死亡的人的样本中含有一系列可能的正丁烷代谢物:正丁醇、2-丁醇、2,3-丁二醇、3-羟基-2-丁酮和2,3-丁二酮。据作者所知,最后三种化合物尚未被提出作为人类正丁烷的代谢物。这些物质可能是通过类似于正己烷和正庚烷的代谢途径产生的...
Purified liquefied petroleum gas (LPG), a mixture of butane, isobutane, and propane, is commonly abused by inhalation. Little is known about the mammalian metabolism of these substances. Metabolism of other hydrocarbons, including n-hexane and cyclohexane, has been studied in vitro using a range of liver preparations, with metabolites analyzed by static headspace techniques... Cyclohexanol and cyclohexanone were formed from cyclohexane and 1-, 2-, and 3-hexanol and 2-hexanone from n-hexane as predicted. Secondary alcohols are found for the other compounds studied, except for propene and isobutane, together with 2-propanone and 2-butanone from propane and n-butane, respectively. Samples from three individuals who died following LPG abuse contained a range of putative n-butane metabolites: n-butanol, 2-butanol, 2,3-butanediol, 3-hydroxy-2-butanone, and 2,3-butanedione. To /the authors/ knowledge, the last three compounds have not been proposed as metabolites of n-butane in man. These might be produced through similar metabolic pathways to those of n-hexane and n-heptane...
来源:Hazardous Substances Data Bank (HSDB)
毒理性
丁烷是一种在室温下无色、可燃的气体。它作为天然气的一个组成部分,从天然气中提炼出来。丁烷被用作燃料、制冷剂和气雾推进剂。经过实验动物吸入暴露后,对丁烷的急性毒性进行了研究。在大鼠中,LC50(4小时)为658毫克/升,而在小鼠中,LC50(2小时)为680毫克/升。在狗中,致死浓度范围从474到592毫克/升。308毫克/升的浓度在25分钟内导致小鼠轻度麻醉,而521毫克/升的暴露在一分钟内产生了类似的效果。吸入后,丁烷...使狗的心肌对肾上腺素诱导的心律失常变得敏感。在现有文献中未找到通过其他给药途径对实验动物进行丁烷急性毒性的报道。在一项为期21天的混合物(丁烷、异丁烷、正戊烷和异戊烷,每种含量25%)吸入毒性研究中,直到最高测试浓度11.8毫克/升,未发现毒性。这项研究是在斯普拉格-道利大鼠上进行的,这些大鼠在三个星期内每天暴露6小时,总共暴露15次。在现有文献中未找到使用纯丁烷的长期研究。在鼠伤寒沙门氏菌TA 1535、TA 1537、TA 1538、TA 98和TA 100的多次测试中,未观察到致突变活性,无论是否添加外源代谢系统。在现有文献中未找到关于致癌性、生殖毒性、致畸性、免疫毒性和过敏的研究。有几份关于人类接触丁烷的报告。挥发性物质滥用的增加,丁烷就是其中之一,增加了与吸入气体相关的突然死亡的风险。导致"高"感觉或死亡的浓度范围已经被注意到非常窄。一名患者使用含有丁烷作为推进剂的烤箱清洁剂导致暂时性肌阵挛。未发现其他物理异常。据报道,一种含有丁烷作为推进剂的气雾剂,当直接喷在皮肤上时,会导致深度冻伤症状。由于丁烷的麻醉效果,检查了来自不同装卸设施和服务站的卡车司机和终端操作员,他们暴露于含有90至92%丁烷、异丁烷、正戊烷和异戊烷的汽油蒸气中。暴露于汽油蒸气的程度远低于已建立的ACGIH阈值(汽油为300 ppm或0.89毫克/升,丁烷为800 ppm或1.9毫克/升)。53名男性炼油工人平均接触丁烷11年(浓度从0.0004毫克/升到0.0178毫克/升),未导致工人的任何临床症状。...总之,据报道,低浓度的丁烷暴露不会对人类造成不利影响。它对人类和实验动物都具有麻醉作用。当高浓度吸入丁烷时,可能会突然死亡。麻醉和致死浓度之间的安全边际似乎非常窄。长期暴露于丁烷已被报道会在中枢神经系统中引起一些症状。在高剂量吸入时,关键效果可能是致死性,以及长期暴露个体的中枢神经系统效应。
n-Butane is a colorless, flammable gas at room temperature. It occurs as a component in natural gas from which it is refined. n-Butane is used as fuel, refrigerant and aerosol propellant. The acute toxicity of n-butane has been studied after inhalation exposure in experimental animals. LC50 (4h) was 658 mg/l in rats and LC50 (2h) was 680 mg/l in mice. In dogs, lethal concentrations ranged from 474 to 592 mg/l. A concentration of 308 mg/l caused light anesthesia in mice within 25 minutes, and an exposure to 521 mg/l had similar effect within one minute. n-Butane ... sensitiz/ed/ the myocardium to epinephrine-induced cardiac arrhythmias in dogs after inhalation. No reports on acute toxicity of n-butane in experimental animals by other administration routes were located in the available literature. In a 21-day inhalation toxicity study of a mixture of n-butane, isobutane, n-penta and isopentane, containing 25% of each, the absence of toxicity was evident up to 11.8 mg/l which was the highest concentration tested. The study was performed in Sprague-Dawley rats which were exposed 6 hours per day over three weeks for a total of 15 exposures. No long-term studies using pure n-butane were located in the available literature. No mutagenic activity was observed in several tests in Salmonella typhimurium strains TA 1535, TA 1537, TA 1538, TA 98 and TA 100 with or without the addition of an exogenous metabolism system. No studies on carcinogenicity, reproduction toxicity and teratogenicity, immunotoxicity or allergy were located in the available literature. Several reports on human exposure to n-butane were available. The increasing abuse of volatile substances, n-butane being among them, increases the risk of sudden death in connection to inhalation of the gas. The range of concentrations that may lead to "high" feelings or to death has been noted to be very narrow. The use of a oven cleaner containing n-butane as propellant has caused transient myoclonus in one patient. No other physical abnormalities were noted. An aerosol spray which contained n-butane as propellant, was reported to cause deep frostbite symptoms ir the skin when sprayed directly on it. Because of the anesthetic effect of n-butane, truck drivers and terminal operators from different loading facilities and service stations were examined for exposure gasoline vapours containing 90 to 92 percent n-butane, isobutane, n-pentane and isopentane. Exposures to the gasoline vapor were substantially lower than the established ACGIH threshold values (300 ppm or 0.89 mg/l for gasoline, and 800 ppm or 1.9 mg/l for n-butane). Occupational exposure of 53 male refinery workers for an average of 11 years to n-butane (concentration varied from 0.0004 mg/l to 0.0178 mg/l) did not cause any clinical symptoms in the workers. ... In conclusion, exposure to low concentrations of n-butane has not been reported to cause adverse effects in humans. It is anesthetic to both humans and experimental animals. Sudden death may occur when n-butane is inhaled at high concentrations. The safety margin between anesthetic and lethal concentrations appears to be very narrow. Chronic exposure to n-butane has been reported to cause some symptoms in the central nervous system. Critical effects might be lethality when inhaled in high doses, and effects on the central nervous system in chronically exposed individuals.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Butane is a simple asphyxiant and causes toxicity by displacing oxygen. It also affects the central nervous system by enhancing glycine receptors and inhibiting N-methyl-d-aspartate (NMDA) receptors. (L1284, A352)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
正丁烷存在于汽油中,对人类可能具有致癌性(2B组)。
n-Butane is found in gasoline, which is possibly carcinogenic to humans (Group 2B). (L135)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
Butane targets the central nervous system and cardiovascular system. Inhalation of butane can cause frostbite which can result in death from asphyxiation and ventricular fibrillation. (L1283, L1284)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
该物质可以通过吸入被身体吸收。
The substance can be absorbed into the body by inhalation.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
吸入研究...在研究中,大鼠和小鼠暴露于致死浓度(27.8-29%)的n-丁烷,结果显示n-丁烷被吸收并分布到各种组织中。经过4小时的呼吸道暴露后,存活的 rats 被处死...在丁烷的浓度中,最高的是肾周脂肪(2086 ppm),其次是大脑(750 ppm)、脾脏(522 ppm)、肝脏(492 ppm)和肾脏(441 ppm)。在暴露于丁烷蒸汽2小时的小鼠中,n-丁烷在大脑中的水平被发现是779 ppm。在大鼠和小鼠中,n-丁烷在大脑中的水平与中枢神经系统抑制的程度相关...尚未有关于n-丁烷蒸汽经皮吸收的报道。然而,由于皮肤接触是短暂的,因为挥发性,所以预计丁烷的经皮渗透不会发生到很大程度。
Inhalation studies ... in which rats and mice were exposed to lethal concn (27.8-29%) revealed that n-butane is absorbed and distributed to various tissues. After 4 hr of respiratory exposure, surviving rats were sacrificed ... concn of butane were ... highest in perinephric fat (2086 ppm), then brain (750 ppm), spleen (522 ppm), liver (492 ppm), and kidney (441 ppm). In mice exposed to 2 hr of butane vapors, the brain levels of n-butane were found to be 779 ppm. In both rats and mice the brain levels of n-butane correlated with the degree of CNS depression ... Dermal absorption of n-butane vapors has not been reported. However, dermal penetration of butane would not be expected to occur to any large extent since skin contact is transient because of volatility.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
由于其挥发性,可以预期丁烷会通过呼气排出。
Because of its volatile nature, elimination of butane by exhalation can be anticipated.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 800 ppm (1900 mg/m3)
-
危险等级:2.1
-
立即威胁生命和健康浓度:1,600 ppm (>10% LEL)
-
危险品标志:F,F+
-
安全说明:S,S16,S9
-
危险类别码:R12
-
WGK Germany:-
-
RTECS号:EJ4200000
-
海关编码:3606100000
-
危险类别:2.1
-
危险标志:GHS02,GHS04
-
危险品运输编号:UN 2037 2.1
-
危险性描述:H220,H280
-
危险性防范说明:P210,P377,P403,P410
-
储存条件:储存注意事项: - 储存于阴凉、通风的易燃气体专用库房。 - 远离火种、热源,库温不宜超过30℃。 - 应与氧化剂、卤素分开存放,切忌混储。 - 采用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 正丁烷 |
| 化学品英文名称: | Butane |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 106-97-8 |
| 分子式: | C 4 H 10 |
| 分子量: | 58.12 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:正丁烷 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第2.1类 易燃气体 |
| 侵入途径: | 吸入 |
| 健康危害: | 主要作用是麻醉和弱刺激。急性中毒:主要表现为头痛、头晕、嗜睡、恶心、酒醉状态,严重者可出现昏迷。慢性影响:出现头痛、头晕、睡眠不佳、易疲倦等症状。 |
| 环境危害: | |
| 燃爆危险: | 本品易燃,具窒息性。 |
| 第四部分:急救措施 |
| 皮肤接触: | |
| 眼睛接触: | |
| 吸入: | 迅速脱离现场至空气新鲜处。注意保暖,呼吸困难时给输氧。呼吸及心跳停止者立即进行人工呼吸和心脏按压术。就医。 |
| 食入: |
| 第五部分:消防措施 |
| 危险特性: | 与空气混合能形成爆炸性混合物,遇明火、高热能引起燃烧爆炸。其蒸气比空气重,能在较低处扩散到相当远的地方,遇火源引着回燃。若遇高热,容器内压增大,有开裂和爆炸的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 切断气源。若不能立即切断气源,则不允许熄灭正在燃烧的气体。喷水冷却容器,可能的话将容器从火场移至空旷处。雾状水、泡沫、二氧化碳。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | -60 |
| 自燃温度(℃): | 287 |
| 爆炸下限[%(V/V)]: | 1.5 |
| 爆炸上限[%(V/V)]: | 8.5 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至上风处,并隔离直至气体散尽,切断火源。建议应急处理人员戴自给式呼吸器,穿一般消防防护服。切断气源,喷雾状水稀释、溶解,将漏出气用排风机送至空旷地方或装设适当喷头烧掉。抽排(室内)或强力通风(室外)。漏气容器不能再用,且要经过技术处理以清除可能剩下的气体。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防静电工作服。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止气体泄漏到工作场所空气中。避免与氧化剂、卤素接触。在传送过程中,钢瓶和容器必须接地和跨接,防止产生静电。搬运时轻装轻卸,防止钢瓶及附件破损。配备相应品种和数量的消防器材及泄漏应急处理设备。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。库温不超过30℃,相对湿度不超过80%。应与氧化剂、卤素分开存放,切忌混储。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中国MAC:未制定标准苏联MAC:300mg/m3美国TWA:ACGIH 800ppm,1900mg/m3 TLVWN: 未制定标准 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 高浓度环境中,佩带供气式呼吸器。 |
| 眼睛防护: | 一般不需特殊防护,高浓度接触时可戴化学安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 一般不需特殊防护,高浓度接触时可戴防护手套。 |
| 其他防护: | 工作现场严禁吸烟。避免长期反复接触。进入罐或其它高浓度区作业,须有人监护。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色气体,有轻微的不愉快气味。 |
| pH: | |
| 熔点(℃): | -138.4 |
| 沸点(℃): | -0.5 |
| 相对密度(水=1): | 0.58 |
| 相对蒸气密度(空气=1): | 2.05 |
| 饱和蒸气压(kPa): | 106.39/O℃ |
| 燃烧热(kJ/mol): | 2653 |
| 临界温度(℃): | 151.9 |
| 临界压力(MPa): | 3.79 |
| 辛醇/水分配系数的对数值: | 无资料 |
| 闪点(℃): | -60 |
| 引燃温度(℃): | 287 |
| 爆炸上限%(V/V): | 8.5 |
| 爆炸下限%(V/V): | 1.5 |
| 分子式: | C 4 H 10 |
| 分子量: | 58.12 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 易溶于水、醇、氯仿。 |
| 主要用途: | 用于有机合成和乙烯制造,仪器校正,也用作燃料等。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、卤素。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:无资料 LC50:658000mg/m3 4小时(大鼠吸入) |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 21012 |
| UN编号: | 1011 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 钢质气瓶;安瓿瓶外普通木箱。 |
| 运输注意事项: | 本品铁路运输时限使用耐压液化气企业自备罐车装运,装运前需报有关部门批准。采用刚瓶运输时必须戴好钢瓶上的安全帽。钢瓶一般平放,并应将瓶口朝同一方向,不可交叉;高度不得超过车辆的防护栏板,并用三角木垫卡牢,防止滚动。运输时运输车辆应配备相应品种和数量的消防器材。装运该物品的车辆排气管必须配备阻火装置,禁止使用易产生火花的机械设备和工具装卸。严禁与氧化剂、卤素等混装混运。夏季应早晚运输,防止日光曝晒。中途停留时应远离火种、热源。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。铁路运输时要禁止溜放。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定;常用危险化学品的分类及标志 (GB 13690-92)将该物质划为第2.1 类易燃气体。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 1 |
| MSDS修改日期: | 年月日 |
制备方法与用途
正丁烷
正丁烷,简称丁烷,分子式C4H10,是一种无色可燃性气体。主要存在于石油和天然气中,具有直链的正丁烷和支链的异丁烷两种异构体。
理化性质:
- 相对密度:0.6012(0℃)、0.5788
- 熔点:-138.4℃
- 沸点:-0.5℃
- 临界温度:152.01℃
- 临界压力:3.797MPa
- 闪点:-60℃
- 折射率(液体,在饱和压力下):1.3259、气体(常压下):1.0013
正丁烷易溶于乙醇、乙醚和氯仿等有机溶剂,微溶于水。与空气混合后可形成爆炸性混合物,爆炸极限为1.6%~8.5%(体积分数)。
生产方法:
用途: 正丁烷除直接用作燃料外,还广泛用于溶剂、致冷剂和有机合成原料。此外,它还可以通过脱氢生成丁烯或丁二烯,或者异构成为异丁烷并进一步脱氢生成异丁烯等。
毒性分级与危险性:
- GRAS(食品添加剂评定机构),ADI未作规定
- 急性毒性吸入:大鼠LC50: 658g/m³ (4小时),小鼠LC50: 680g/m³ (2小时)
- 爆炸物危险特性:与空气混合明火或受热可爆炸
- 可燃性危险特性:明火或受热可燃烧,产生刺激烟雾
储存与运输: 库房应保持通风、低温干燥;轻装轻卸,并需与其他助燃气体钢瓶分开存放。
急性毒性吸入 - 大鼠LC50: 658,000毫克/立方米/4小时; 吸入 - 小鼠LC50: 680,000毫克/立方米/2小时
爆炸物危险特性与空气混合明火、受热可爆
可燃性危险特性明火、受热可燃;燃烧产生刺激烟雾
储运特性库房通风低温干燥;轻装轻卸;与氧气、空气等助燃气体钢瓶分开存放
灭火剂 职业标准TWA 1900毫克/立方米; STEL 2350毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 正戊烷 pentane 109-66-0 C5H12 72.1503 环丁烷 cyclobutane 287-23-0 C4H8 56.1075 丙烷 propane 74-98-6 C3H8 44.0965 正己烷 hexane 110-54-3 C6H14 86.1772 环戊烷 Cyclopentane 287-92-3 C5H10 70.1344 2-甲基丁烷 methylbutane 78-78-4 C5H12 72.1503 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 正戊烷 pentane 109-66-0 C5H12 72.1503 丙烷 propane 74-98-6 C3H8 44.0965 正己烷 hexane 110-54-3 C6H14 86.1772 2-甲基丁烷 methylbutane 78-78-4 C5H12 72.1503 环戊烷 Cyclopentane 287-92-3 C5H10 70.1344
反应信息
-
作为反应物:参考文献:名称:Oxidation of Butane to Maleic Anhydride on Unmodified and Modified Vanadium-Phosphorus Catalysts摘要:
研究了改性钒-磷催化剂的性质,并与未改性催化剂在丁烷氧化中的性能进行了比较。通过向催化剂晶格中引入钴,实现了最大的促进效果。在90%的转化率下,顺丁烯二酸酐的产量达到了59摩尔百分比。与未改性催化剂相比,用钴改性的催化剂将丁烷氧化和顺丁烯二酸酐形成的特定速率提高了三倍。催化剂的特定活性按以下顺序降低:Co > U ~ Ce > V-P-O > K ~ Mo。
DOI:10.1135/cccc19950457 -
作为产物:参考文献:名称:Calingaert bei Zartman; Adkins, Journal of the American Chemical Society, 1932, vol. 54, p. 3400摘要:DOI:
-
作为试剂:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: S: SVol.4a/b, 1.1.4.3.2, page 19 - 22摘要:DOI:
文献信息
-
一类烷基芳胺类化合物及其制备方法
-
Mechanistic Interrogation of Alkyne Hydroarylations Catalyzed by Highly Reduced, Single-Component Cobalt Complexes作者:Benjamin A. Suslick、T. Don TilleyDOI:10.1021/jacs.0c04072日期:2020.6.24complexes. Studies of the stoichiometric reaction of Co(I) or Co(II) precursors with CyMgCl implicated catalyst initiation via a β-H elimination/deprotonation pathway. The resulting single-component Co(-I) complex is proposed as the direct precatalyst. Michaelis-Menten enzyme kinetic studies provide mechanistic details regarding the catalytic dependence on substrate. The (N-aryl)aryl ethanimine substrate以前曾报道过用于炔烃的邻位加氢芳基化的高反应性催化剂是由格氏试剂活化 CoBr2 引起的,但活性钴物种的操作机制和身份尚未确定。使用可分离的还原 Co 配合物对相关系统进行了机械分析,包括 (N-芳基) 芳基乙胺与二苯乙炔的加氢芳基化。Co(I) 或 Co(II) 前体与 CyMgCl 的化学计量反应的研究涉及通过 β-H 消除/去质子化途径引发的催化剂。由此产生的单组分 Co(-I) 复合物被提议作为直接预催化剂。Michaelis-Menten 酶动力学研究提供了关于对底物的催化依赖性的机制细节。(N-芳基)芳基乙胺底物表现出类似饱和的行为,而炔烃表现出复杂的依赖性;速率抑制和促进取决于炔烃与亚胺的相对浓度。芳基 CH 键的活化仅发生在配位炔烃的存在下,这表明协同金属化-去质子化 (CMD) 机制的运行。小的初级同位素效应与决定速率的 CH 裂解一致。由相同的 Co(-I) 活性物质催化的非循环烯烃异构化似乎是观察到的
-
Gas-phase reactions of iron(1-) and cobalt(1-) with simple thiols, sulfides, and disulfides by Fourier-transform mass spectrometry作者:L. Sallans、K. R. Lane、B. S. FreiserDOI:10.1021/ja00185a013日期:1989.2products, H-Fesup minus}}-SH and Fesup minus}}-SH. Some of the thermochemical data derived from this study include Ddegree}(Msup minus}}-S) > 103 kcal/mol and Ddegree}(Msup minus}}-SH) = 83 plus minus}9 kcal/mol. Finally, a brief survey of the reactivity of Vsup minus}}, Crsup minus}}, and Mosup minus}} with selected organosulfur compounds is also reported. 79 refs., 3 figs., 7 tabs发现 Fesup minus}} 和 Cosup minus}} 会与简单的硫醇、硫化物和二硫化物反应。由这些金属阴离子 Msup minus}} 和硫醇形成的主要反应产物包括 MSsup minus}}、MSHsup minus}} 和 MSHsub 2}sup minus}} 和提出了一种涉及金属初始插入弱 CS 键的机制。类似地,CS 插入是与硫化物和二硫化物反应的主要攻击模式,类似于观察到的金属阳离子反应。碰撞诱导解离用于支持主要产物 H-Fesup minus}}-SH 和 Fesup minus}}-SH 的拟议结构。本研究得出的一些热化学数据包括 Ddegree}(Msup minus}}-S) > 103 kcal/mol 和 Ddegree}(Msup minus}}-SH) = 83 plus减去}9 kcal/mol。最后,还报告了
-
Synthesis of Al-MTW with low Si/Al ratios by combining organic and inorganic structure directing agents
-
Investigation of the Mechanism of <i>n</i>-Butane Oxidation on Vanadium Phosphorus Oxide Catalysts: Evidence from Isotopic Labeling Studies作者:Bin Chen、Eric J. MunsonDOI:10.1021/ja010285v日期:2002.2.1minimize total oxidation products such as CO and CO(2), the amounts of ethylene and carbon oxides produced from fully (13)C-labeled butane were almost equal. This strongly suggests that the total oxidation of n-butane on VPO catalysts involves the oxidation and abstraction of the two methyl groups of n-butane, and the two methylene groups of n-butane form ethylene. An organometallic mechanism is proposed由磷酸钒 (VPO) 催化的正丁烷选择性氧化为马来酸是当今工业中使用的最复杂的部分氧化反应之一。文献中提出了许多反应机理,其中许多都以丁烯、丁二烯和呋喃作为反应中间体。我们开发了一个实验方案来研究该反应的机理,其中 (13) C 同位素标记的正丁烷流过催化剂床,并使用 (13) C 核磁共振光谱分析反应产物。该协议近似于在工业反应器中发现的条件,而不需要过多的同位素标记材料。当 [1,4-(13)C]n-丁烷在 VPO 催化剂上反应生成马来酸和丁二烯时,在 1,4 和 2 中均观察到同位素标记,丁二烯和马来酸的 3 个位置。对于马来酸中的 2,3:1,4 位置,标记加扰的比率通常为 1:20。对于丁二烯,标记加扰的比率一直要高得多,2,3:1,4 位置为 2:3。由于马来酸和丁二烯之间的标记量存在差异,丁二烯不太可能是典型工业条件下正丁烷转化为马来酸酐的主要反应中间体。乙烯总是被观察到作为
表征谱图
-
氢谱1HNMR
-
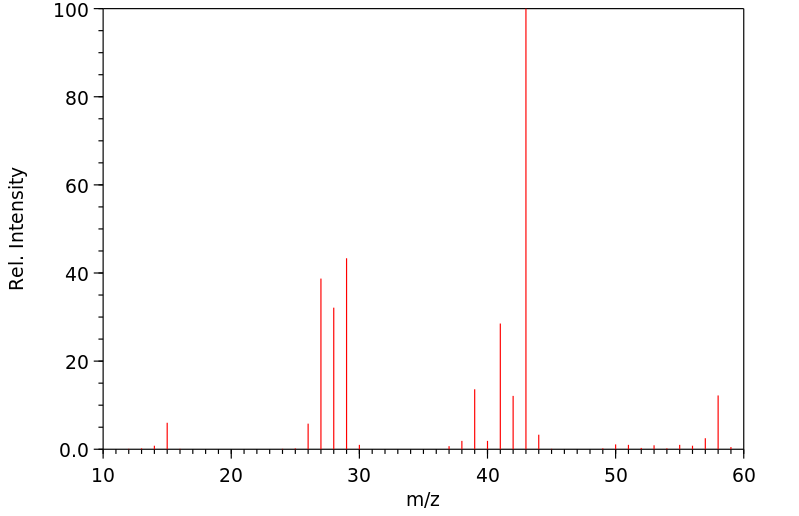
质谱MS
-
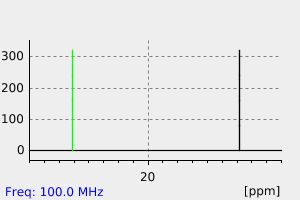
碳谱13CNMR
-
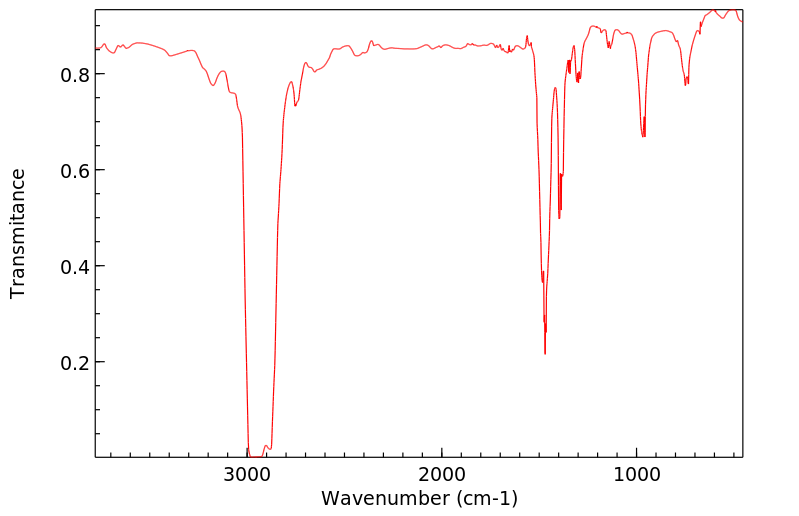
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷