4-氯-2-甲氧基苯胺 | 93-50-5
中文名称
4-氯-2-甲氧基苯胺
中文别名
2-氨基-5-氯苯甲醚;2-甲氧基-4-氯苯胺
英文名称
4-chloro-o-anisidine
英文别名
4-chloro-2-methoxyaniline;4-chloro-2-methoxy-phenylamine;2-methoxy-4-chloroaniline;2-methoxy-4-chlorobenzenamine
CAS
93-50-5
化学式
C7H8ClNO
mdl
MFCD06801386
分子量
157.6
InChiKey
WOXLPNAOCCIZGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:52°C
-
沸点:260°C (rough estimate)
-
密度:1.1760 (rough estimate)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:35.2
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
海关编码:2922299090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:保持冷静
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Chloro-2-methoxyaniline
Synonyms: 2-Amino-5-chloroanisole
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Chloro-2-methoxyaniline
CAS number: 93-50-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H8ClNO
Molecular weight: 157.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Chloro-2-methoxyaniline
Synonyms: 2-Amino-5-chloroanisole
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Chloro-2-methoxyaniline
CAS number: 93-50-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H8ClNO
Molecular weight: 157.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-5-氯苯酚 2-amino-5-chlorophenol 28443-50-7 C6H6ClNO 143.573 邻甲氧基苯胺 2-methoxy-phenylamine 90-04-0 C7H9NO 123.155 5-氯-2-硝基苯甲醚 1-chloro-3-methoxy-4-nitrobenzene 6627-53-8 C7H6ClNO3 187.583 3-氯苯甲醚 1-chloro-3-methoxy-benzene 2845-89-8 C7H7ClO 142.585 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-chloro-2-methoxy-N,N-dimethyl-aniline 35122-85-1 C9H12ClNO 185.653 —— 4-chloro-N-(4-chlorophenyl)-2-methoxyaniline —— C13H11Cl2NO 268.142 —— 4-Chloro-N-phenyl-o-anisidine 97026-57-8 C13H12ClNO 233.697 N-(4-氯-2-甲氧基苯基)乙酰胺 4'-chloro-o-acetanisidide 86412-57-9 C9H10ClNO2 199.637 3-氯苯甲醚 1-chloro-3-methoxy-benzene 2845-89-8 C7H7ClO 142.585 1-(4-氯-2-甲氧基苯基)哌嗪 1-(4-chloro-2-methoxyphenyl)-piperazine 89989-01-5 C11H15ClN2O 226.706 N-(4-氯-6-甲氧基苯基)-2,2-二甲基丙酰胺 N-(4-chloro-2-methoxyphenyl)-2,2-dimethylpropanamide 113137-29-4 C12H16ClNO2 241.718 —— 4-chloro-2-methoxy-5-nitroaniline 22459-72-9 C7H7ClN2O3 202.597
反应信息
-
作为反应物:描述:4-氯-2-甲氧基苯胺 在 tetrafluoroboric acid 、 2-甲基-2-硝基丙烷 、 potassium iodide 作用下, 以 水 、 乙腈 为溶剂, 以204 mg的产率得到5-氯-2-碘苯甲醚参考文献:名称:通过连续一锅钼催化还原/Sandmeyer 反应从硝基芳烃直接合成卤代芳烃摘要:在此,我们报道了在连续的一锅操作中从硝基芳烃直接合成各种官能化芳香族溴化物、氯化物、碘化物和氟化物。该方案基于耐空气和耐湿气的二氧钼催化硝基芳香族化合物的还原,采用频哪醇作为还原剂,从而实现后续的重氮化和卤化步骤。该方法代表了直接从硝基芳族化合物合成卤代芳族化合物的经济、实用和替代程序。DOI:10.1039/d3ob01187a
-
作为产物:描述:参考文献:名称:Brand, Journal fur praktische Chemie (Leipzig 1954), 1903, vol. <2> 67, p. 152,153,163摘要:DOI:
文献信息
-
一种具有抗肿瘤活性的苯基磺胺类化合物及 其制备和应用申请人:浙江树人学院公开号:CN108658943B公开(公告)日:2021-04-30本发明公开一种新型的苯基磺胺类化合物及其制备方法和应用。本发明的化合物结构如(I)所示,其中,R1为H、CH3、CH2 中的一种;R2为H、F、Cl、Br、 、CF3、NO2、O 、萘基、叔丁基、2,4‑二氟、3,5‑二氟中的一种;R3为 、O 、 、O 中的一种;R4和R5为H或 中的一种。其制备步骤包括:将结构式如(II)所示的苯胺嘧啶衍生物溶于一定的溶剂中,然后加入一定量的结构式如(III)所示的苯磺酰叠氮化合物或其同系物,在铑试剂和银添加剂的催化下,在合适的温度下进行反应,待反应结束后,经过后处理并用甲醇重结晶得到产品。本发明的苯基磺胺类化合物在抗肿瘤细胞方面存在抑制功能,具有一定的应用前景。
-
Diamine derivatives申请人:Ohta Toshiharu公开号:US20050020645A1公开(公告)日:2005-01-27A compound represented by the general formula (1): Q 1 -Q 2 -T 0 -N(R 1 )-Q 3 -N(R 2 )-T 1 -Q 4 (1) wherein R 1 and R 2 are hydrogen atoms or the like; Q 1 is a saturated or unsaturated, 5- or 6-membered cyclic hydrocarbon group which may be substituted, or the like; Q 2 is a single bond or the like; Q 3 is a group in which Q 5 is an alkylene group having 1 to 8 carbon atoms, or the like; and T 0 and T 1 are carbonyl groups or the like; a salt thereof, a solvate thereof, or an N-oxide thereof. The compound is useful as an agent for preventing and/or treating cerebral infarction, cerebral embolism, myocardial infarction, angina pectoris, pulmonary infarction, pulmonary embolism, Buerger's disease, deep venous thrombosis, disseminated intravascular coagulation syndrome, thrombus formation after valve or joint replacement, thrombus formation and reocclusion after angioplasty, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), thrombus formation during extracorporeal circulation, or blood clotting upon blood drawing.通用式(1)表示的化合物: Q1-Q2-T0-N(R1)-Q3-N(R2)-T1-Q4(1) 其中R1和R2是氢原子或类似物;Q1是饱和或不饱和的、5-或6-成员环烃基,可以被取代,或类似物;Q2是单键或类似物;Q3是一个基团,其中Q5是具有1至8个碳原子的烷基基团,或类似物;T0和T1是羰基团或类似物;其盐、溶剂合物或N-氧化物。 该化合物可用作预防和/或治疗脑梗死、脑栓塞、心肌梗死、心绞痛、肺梗死、肺栓塞、布尔格病、深静脉血栓形成、弥散性血管内凝血综合征、瓣膜或关节置换后的血栓形成、血管成形术后的血栓形成和再闭塞、全身性炎症反应综合征(SIRS)、多器官功能障碍综合征(MODS)、体外循环期间的血栓形成,或抽血时的血液凝结。
-
[EN] AZEPANYL-DERIVATIVES AND PHARMACEUTICAL COMPOSITIONS COMPRISING THE SAME WITH ANTIPARASITIC ACTIVITY<br/>[FR] DÉRIVÉS D'AZÉPANYLE ET COMPOSITIONS PHARMACEUTIQUES À ACTIVITÉ ANTI-PARASITAIRE LES CONTENANT申请人:MERCK PATENT GMBH公开号:WO2016000827A1公开(公告)日:2016-01-07The present invention provides compounds of Formula (i). Furthermore, pharmaceutical compositions are provided comprising at least one compound of Formula (i), for the treatment of parasitic diseases including malaria, as well as neurodegenerative diseases.本发明提供了化合物的结构式(i)。此外,提供了包含至少一种结构式(i)化合物的药物组合物,用于治疗包括疟疾在内的寄生虫病以及神经退行性疾病。
-
[EN] METHODS AND COMPOUNDS FOR RESTORING MUTANT p53 FUNCTION<br/>[FR] MÉTHODES ET COMPOSÉS POUR LA RESTAURATION DE LA FONCTION DU P53 MUTANT申请人:PMV PHARMACEUTICALS INC公开号:WO2021061643A1公开(公告)日:2021-04-01Mutations in oncogenes and tumor suppressors contribute to the development and progression of cancer. The present disclosure describes compounds and methods to recover wild-type function to p53 mutants. The compounds of the present invention can bind to mutant p53 and restore the ability of the p53 mutant to bind DNA and activate downstream effectors involved in tumor suppression. The disclosed compounds can be used to reduce the progression of cancers that contain a p53 mutation.
-
[EN] BENZOTHIADIAZINE COMPOUNDS<br/>[FR] COMPOSÉS BENZOTHIADIAZINE申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2017098421A1公开(公告)日:2017-06-15The invention is directed to substituted benzothiadiazine derivatives. Specifically, the invention is directed to compounds according to Formula (I):wherein R, R1, R2, R3, R4 and R5 are as defined herein. The compounds of the invention are inhibitors of CD73 and can be useful in the treatment of cancer, pre-cancerous syndromes and diseases associated with CD73 inhibition, such as AIDS, autoimmune diseases, infections, atherosclerosis, and ischemia-reperfusion injury. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting CD73 activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
表征谱图
-
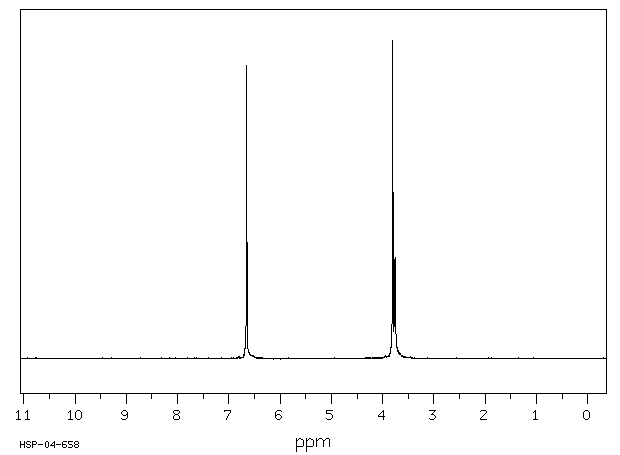
氢谱1HNMR
-
质谱MS
-
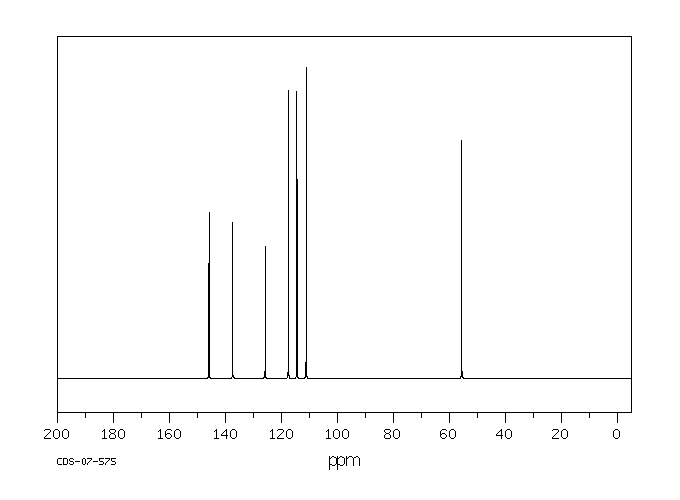
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯