1,8-二碘辛烷 | 24772-63-2
中文名称
1,8-二碘辛烷
中文别名
亚辛基二碘化物
英文名称
1,8-diiodooctane
英文别名
——
CAS
24772-63-2
化学式
C8H16I2
mdl
MFCD00001106
分子量
366.024
InChiKey
KZDTZHQLABJVLE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:16-21 °C
-
沸点:167-169 °C/6 mmHg (lit.)
-
密度:1.84 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:与甲醇混溶。
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。请避免接触光线和氧化物。
计算性质
-
辛醇/水分配系数(LogP):5.7
-
重原子数:10
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:Xi
-
安全说明:S26
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:2810
-
海关编码:2903399090
-
包装等级:III
-
危险类别:6.1(b)
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:| 0-6℃ |
SDS
模块 1. 化学品
1.1 产品标识符
: 1,8-二碘辛烷
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 3)
眼睛刺激 (类别 2B)
慢性水生毒性 (类别 4)
2.2 GHS 标记要素,包括预防性的陈述
象形图 无
警示词 警告
危险申明
H316 引起轻微皮肤刺激。
H320 造成眼刺激。
H413 可能对水生生物产生长期持续的有害影响。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P273 避免释放到环境中。
事故响应
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P337 + P313 如仍觉眼睛刺激:求医/就诊。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
: C8H16I2
分子式
: 366.02 g/mol
分子量
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
无数据资料
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
用惰性吸附材料吸收并当作危险废物处理。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
对光线敏感
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
167 - 169 °C 在 8 hPa
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.84 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 5.033 -
前面的数据或数据判读是根据定量结构活性关系(QSAR)确定的。
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强碱
10.6 危险的分解产物
在着火情况下,会分解生成有害物质。 - 碳氧化物, 碘化氢
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
轻度的皮肤刺激 前面的数据或数据判读是根据定量结构活性关系(QSAR)确定的。
眼睛刺激或腐蚀
轻度的眼睛刺激 前面的数据或数据判读是根据定量结构活性关系(QSAR)确定的。
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:雷尼镍:一种有效的有机卤化物还原脱卤试剂摘要:Raney镍是一种有效的试剂,用于实现有机卤化物的化学选择性还原脱卤。在这种实验条件下,氟化物和烯烃卤化物不发生反应。DOI:10.1055/s-2001-12325
-
作为产物:参考文献:名称:限于动态超分子主体的二卤化物的高度选择性自由基单还原。摘要:(的烷基二卤化物客人减少2 - 5和7)三烷基硅烷与(R 3在水溶性宿主进行的SiH)1来调查快速自由基反应限制效应(ķ ≥10 3 米-1 小号-1)。在温和条件下,对初级和次级二卤化物客人观察到单还原产物的高选择性(> 95%)。结果突出了主客体络合速率对调节自由基反应中产物选择性的重要性。DOI:10.1002/chem.202004953
文献信息
-
Probing the Existence of a Metastable Binding Site at the β<sub>2</sub>-Adrenergic Receptor with Homobivalent Bitopic Ligands作者:Birgit I. Gaiser、Mia Danielsen、Emil Marcher-Rørsted、Kira Røpke Jørgensen、Tomasz M. Wróbel、Mikael Frykman、Henrik Johansson、Hans Bräuner-Osborne、David E. Gloriam、Jesper Mosolff Mathiesen、Daniel Sejer PedersenDOI:10.1021/acs.jmedchem.9b00595日期:2019.9.12development of bitopic ligands aimed at targeting the orthosteric binding site (OBS) and a metastable binding site (MBS) within the same receptor unit. Previous molecular dynamics studies on ligand binding to the β2-adrenergic receptor (β2AR) suggested that ligands pause at transient, less-conserved MBSs. We envisioned that MBSs can be regarded as allosteric binding sites and targeted by homobivalent bitopic在本文中,我们报道了针对同一受体单元内正构结合位点(OBS)和亚稳结合位点(MBS)的双配位配体的开发。先前对配体与β2-肾上腺素受体(β2AR)结合的分子动力学研究表明,配体在瞬时的,保守性较低的MBSs处暂停。我们设想,MBS可以被视为变构结合位点,并由连接两个相同药效基团的同双价双位配体靶向。基于拮抗剂(S)-普萘洛尔对接至OBS和MBS中来设计此类配体并进行合成。药理学特征显示,与(S)-阿普萘洛尔相比,配体具有相似的效价和亲和力,β2/β1AR选择性略有增加,和/或β2AR的离解速率大大降低。截短的双位配体表明亚稳态药效团的主要贡献是与β2AR的疏水相互作用,而单独的接头降低了正构片段的效力。总而言之,该研究强调了靶向MBS改善配体药理作用的潜力。
-
Copper(I)-Catalyzed Asymmetric Alkylation of Unsymmetrical Secondary Phosphines作者:Shuai Zhang、Jun-Zhao Xiao、Yan-Bo Li、Chang-Yun Shi、Liang YinDOI:10.1021/jacs.1c04112日期:2021.7.7enantioselectivity in this reaction is attributed to the high performance of the unique Cu(I)-(R,RP)-TANIAPHOS complex in asymmetric induction. Finally, one monophosphine and two bisphosphines prepared by the present reaction are employed as efficient chiral ligands to afford three structurally diversified Cu(I) complexes, which demonstrates the synthetic utility of the present methodology.铜 (I) 催化的 HPAr 1 Ar 2与烷基卤化物的不对称烷基化被发现,它以通常高产率和对映选择性提供了一系列P-立体膦。亲电子卤代烷具有广泛的底物范围,包括烯丙基溴、炔丙基溴、苄基溴和烷基碘。此外,11 个不对称的二芳基膦 (HPAr 1 Ar 2 ) 可作为有能力的亲核试剂。该方法还成功地应用于催化不对称双烷基化和三烷基化,并以中等的非对映选择性和优异的对映选择性获得了相应的产物。一些31P NMR 实验表明,体积大的 HPPhMes 对 Cu(I)-双膦配合物的竞争配位能力较弱,因此化学计量的 HPAr 1 Ar 2 的存在不会显着影响对映选择性。因此,该反应的高对映选择性归因于独特的 Cu(I)-( R , R P )-TANIAPHOS 络合物在不对称诱导中的高性能。最后,通过本反应制备的一种单膦和两种双膦用作有效的手性配体,以提供三种结构多样化的 Cu(I) 配合物,这证明了本方法的合成效用。
-
Dynamic neighbouring participation of nitrogen lone pairs on the chromogenic behaviour of trans-bis(salicylaldiminato)Pt(<scp>ii</scp>) coordination platforms作者:Takumi Hashimoto、Kanako Fukumoto、Ngoc Ha-Thu Le、Tatsuya Matsuoka、Soichiro Kawamorita、Naruyoshi Komiya、Takeshi NaotaDOI:10.1039/c6dt04005e日期:——The participation of neighbouring nitrogen lone pairs in the chromogenic control of trans-bis(salicylaldiminato)Pt(II) platforms was examined, using newly designed Pt analogues bearing salicylaldehyde hydrazone ligands. A series of non-vaulted and vaulted Pt complexes (1–5) with salicylaldehyde hydrazones as trans-coordinated bidentate ligands were synthesized and characterized with regard to the chromogenic使用新设计的带有水杨醛配体的Pt类似物,检查了相邻的氮孤对在反式双-(salicylaldiminato)Pt(II)平台的生色控制中的参与。一系列的非拱形和拱形Pt配合物(1-5)与水杨醛腙为反式配位的二齿配体被合成和表征相对于的发色行为反式-双(水杨醛)铂(II)协调平台。X射线衍射和2D NMR数据表明,在非拱形N-单甲基络合物1的情况下,,由于分子内氢键引起的构象固定,相邻的N(2)个孤对在d-π共轭的反式-bis(salicylaldiminato)Pt(II)平台中显着参与。相比之下,N,N-二甲基类似物2的孤对由于其高的构象迁移率,对d-π共轭的延伸贡献不大。发现配合物1-5在溶液状态下具有结构相关的发色性质,因此N-甲基,短拱形配合物1和3的吸收光谱相对于N,N-二甲基,长拱形类似物2和5表现出明显的七色移,其光谱与反式-bis(salicylaldiminato)Pt(I
-
Synthese von α-Halogen-ω-alkylthio-alkanen und α,ω-Bisalkylthio-alkanen作者:Elke AnklamDOI:10.1055/s-1987-28097日期:——Synthesis of α-Halogeno-Ï-alkylthioalkanes and α,Ï-Bisalkylthioalkanes The reaction of α,Ï-dihalogenated alkanes [X(CH2)nX,,n = 3 - 10]with alkylthiolates affords α-halogeno-Ï-alkylthioalkanes 1 as well as α,Ï-bisalkylthioalkanes 2. Products 1 (40 examples) and 2 (22 examples) are easily isolated in good yields by flash chromatography.
-
[EN] METHODS AND COMPOSITIONS FOR TARGETED THERAPEUTICS<br/>[FR] MÉTHODES ET COMPOSITIONS POUR UN USAGE THÉRAPEUTIQUE CIBLÉ申请人:MEDIVATION TECHNOLOGIES INC公开号:WO2017019830A1公开(公告)日:2017-02-02This disclosure describes compositions and methods for delivering and localizing repair cells to therapeutic targets.这份披露描述了将修复细胞传递和定位到治疗靶点的组合物和方法。
表征谱图
-
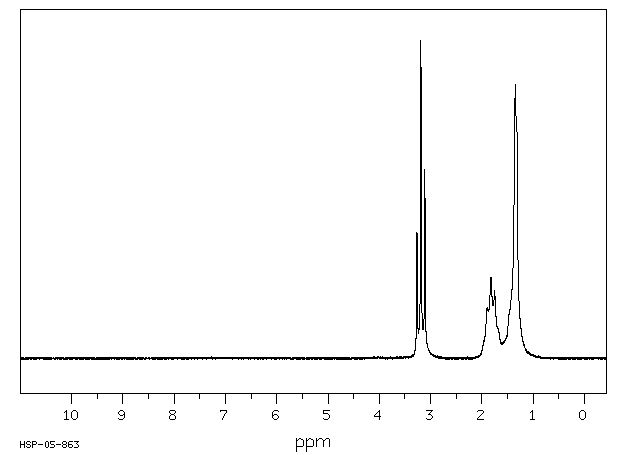
氢谱1HNMR
-
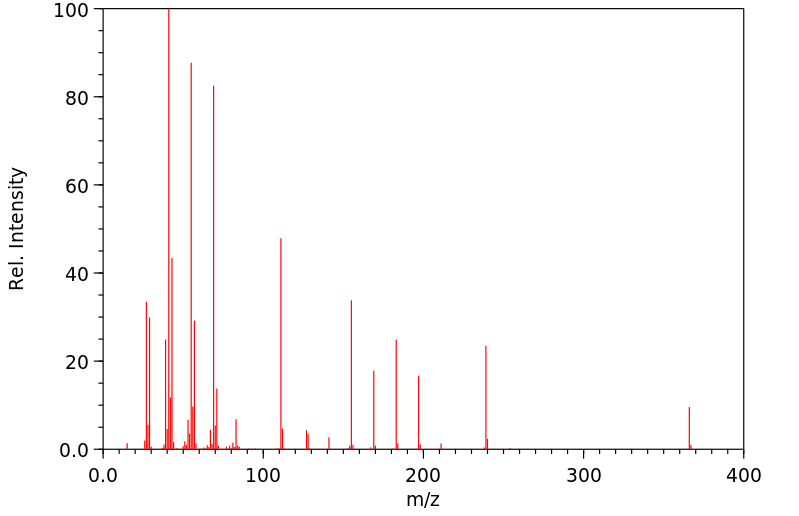
质谱MS
-
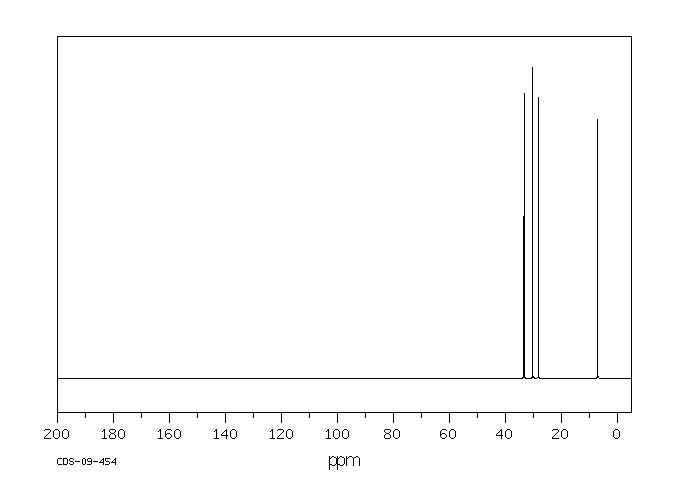
碳谱13CNMR
-
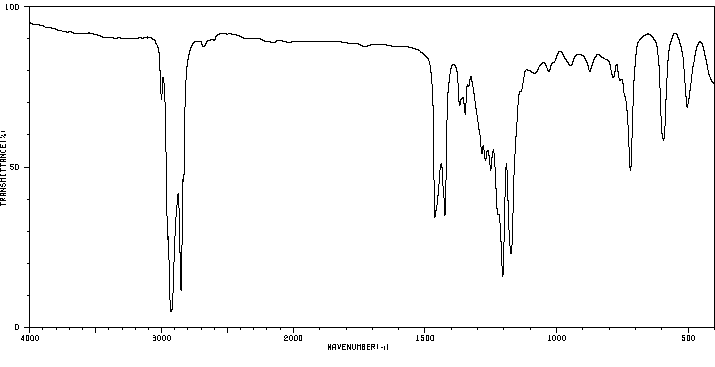
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物