炔丙基脲 | 6089-09-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:54-57 °C(lit.)
-
沸点:110 °C30 mm Hg(lit.)
-
密度:1.1133 (rough estimate)
-
闪点:75 °C
-
LogP:0.402 (est)
-
稳定性/保质期:
在常温常压下稳定,这种物质呈现灰黄色至白色结晶状。
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2916190090
-
危险品运输编号:UN 3261 8/PG 2
-
危险类别:8
-
RTECS号:SC4751000
-
包装等级:III
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H314
-
储存条件:存储条件:2-8℃,避光,置于阴凉干燥处,密封保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 4-戊炔酸
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤腐蚀 (类别 1B)
严重眼睛损伤 (类别 1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H314 造成严重皮肤灼伤和眼损伤。
警告申明
预防措施
P260 不要吸入粉尘或烟雾。
P264 操作后彻底清洁皮肤。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
事故响应
P301 + P330 + P331 如果吞咽:漱口,不要催吐。
P303 + P361 + P353 如果皮肤(或头发)接触:立即除去/脱掉所有沾污的衣物,用水清洗皮肤/淋
浴。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P363 沾污的衣服清洗后方可再用。
安全储存
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C5H6O2
分子式
: 98.10 g/mol
分子量
组分 浓度或浓度范围
Pent-4-ynoic acid
<=100%
化学文摘登记号(CAS 6089-09-4
No.) 228-028-0
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
立即脱掉被污染的衣服和鞋。 用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
该物质对粘膜组织和上呼吸道、眼睛和皮肤破坏巨大。, 痉挛,发炎,咽喉肿痛, 痉挛,发炎,支气管炎, 肺炎,
肺水肿, 灼伤感:, 咳嗽, 喘息, 喉炎, 呼吸短促, 头痛, 恶心, 呕吐,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
建议的贮存温度: 2 - 8 °C
对光线敏感 充气保存 对空气敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 54 - 57 °C
f) 沸点、初沸点和沸程
110 °C 在 40 hPa
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 静脉内的 - 小鼠 - 28 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 该物质对组织、粘膜和上呼吸道破坏力强
摄入 如服入是有害的。 引致灼伤。
皮肤 通过皮肤吸收可能有害。 引起皮肤灼伤。
眼睛 引起眼睛灼伤。
接触后的征兆和症状
该物质对粘膜组织和上呼吸道、眼睛和皮肤破坏巨大。, 痉挛,发炎,咽喉肿痛, 痉挛,发炎,支气管炎, 肺炎,
肺水肿, 灼伤感:, 咳嗽, 喘息, 喉炎, 呼吸短促, 头痛, 恶心, 呕吐,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: SC4751000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 3261 国际海运危规: 3261 国际空运危规: 3261
14.2 联合国运输名称
欧洲陆运危规: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (Pent-4-ynoic acid)
国际海运危规: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (Pent-4-ynoic acid)
国际空运危规: CorrOSiVE solid, acidic, organic, n.o.s. (Pent-4-ynoic acid)
14.3 运输危险类别
欧洲陆运危规: 8 国际海运危规: 8 国际空运危规: 8
14.4 包裹组
欧洲陆运危规: II 国际海运危规: II 国际空运危规: II
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
4-戊炔酸可用作有机合成中间体,广泛应用于医药、材料科学和农业等领域。它能够作为制备抗菌药、抗炎药、导电材料及光电材料的中间体。在农业领域,该化合物还可用作植物生长调节剂的原料,通过调节植物的生长和发育过程,提高农作物的产量和品质。
合成将20%氢氧化钠溶液(200g)与含有戊烯酸的甲苯(100g)溶液一同加入500ml四颈烧瓶中,在室温下进行反应。逐滴加总量并搅拌20分钟后,待反应温度升至60°C时通过气相色谱法确认反应完全。随后冷却分离液体,并去除有机层。
在20~40°C的条件下,向水层中缓慢滴加10%硫酸(600g,0.612mol),并在搅拌下进行操作。使用混合溶剂(甲苯200g+四氢呋喃100g)提取两次,收集300g有机层并减压蒸馏,得到50g标题化合物(产率84%),为浅棕色晶体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 戊-4-炔酸甲酯 methyl pent-4-ynoate 21565-82-2 C6H8O2 112.128 4-戊炔-1-醇 pent-1-yn-5-ol 5390-04-5 C5H8O 84.1179 —— 4-pentyn-1-al 18498-59-4 C5H6O 82.102 戊-4-酸乙酯 ethyl 4-pentynoate 63093-41-4 C7H10O2 126.155 4-戊烯酸 4-pentenoic acid 591-80-0 C5H8O2 100.117 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-己炔酸 hex-4-ynoic acid 41143-12-8 C6H8O2 112.128 —— 5-chloro-4-pentynoic acid 79054-21-0 C5H5ClO2 132.546 5-溴-1-戊炔酸 5-Bromo-1-pentynoic acid 58065-03-5 C5H5BrO2 176.997 戊-4-炔酸甲酯 methyl pent-4-ynoate 21565-82-2 C6H8O2 112.128 —— 7-hydroxyhept-4-ynoic acid 165619-45-4 C7H10O3 142.155 —— heptane-4,6-diynoic acid 19444-14-5 C7H6O2 122.123 壬-4-炔酸 non-4-ynoic acid 88663-39-2 C9H14O2 154.209 —— 4-decynoic acid 16900-59-7 C10H16O2 168.236 —— decane-4,6-diynoic acid 91061-07-3 C10H12O2 164.204 —— deca-4,6-diynedioic acid 5714-92-1 C10H10O4 194.187 正戊酸 n-Pentanoic acid 109-52-4 C5H10O2 102.133 —— oct-7-en-4-ynoic acid 810668-39-4 C8H10O2 138.166 —— Docosa-4,7,10,13,16,19-hexainsaeure 31820-15-2 C22H20O2 316.4 —— 4-pentadecynoic acid 207132-49-8 C15H26O2 238.37 4-戊炔-1-醇 pent-1-yn-5-ol 5390-04-5 C5H8O 84.1179 —— Nona-4,6,8-triinsaeure 51193-81-8 C9H6O2 146.145 丁二酸 succinic acid 110-15-6 C4H6O4 118.089 戊-4-酸乙酯 ethyl 4-pentynoate 63093-41-4 C7H10O2 126.155 —— Dodeca-4,6,8-triyn-1,12-dioic acid 288264-92-6 C12H10O4 218.209 4-戊烯酸 4-pentenoic acid 591-80-0 C5H8O2 100.117 —— docosa-4,7,10,13,16-pentaynoic acid 16326-33-3 C22H24O2 320.431 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Au2O3 as a Stable and Efficient Catalyst for the Selective Cycloisomerization of γ-Acetylenic Carboxylic Acids to γ-Alkylidene-γ-Butyrolactones摘要:展示了市售金氧化物(Au2O3)作为催化剂,在通过一种通用、高效且简便的过程,将带有羧酸的炔烃环化为相应γ-烷叉基-γ-丁内酯中的高潜力。该反应表现出高度化学选择性、区域选择性和立体选择性。5-内型环化和反式金化是Au2O3催化剂的普遍趋势。DOI:10.1055/s-2008-1032108
-
作为产物:参考文献:名称:Neuroprotectin D1/Protectin D1 及其阿司匹林触发立体异构体的立体控制全合成摘要:Neuroprotectin D1/Protectin D1 是一种从二十二碳六烯酸生物合成衍生的强效抗炎、促分解和神经保护脂质介质,通过全有机合成以对映体纯形式制备。合成策略是高度立体控制和收敛的,以缩水甘油起始材料的环氧化物开环为特征,以引入 10( R ) 和 17( S ) 羟基。通过炔前体的顺式还原来确保所需的烯烃Z几何结构,而在末端引入共轭E、E、Z三烯,以最小化Z / E异构化。相同的策略也用于具有 17( R )-立体化学的阿司匹林触发的神经保护素 D1/保护素 D1 的全合成。用所报告的方法获得的合成化合物与内源性材料相匹配,并有助于建立完整的立体化学。DOI:10.1016/j.tetlet.2012.01.032
-
作为试剂:描述:2,2-dimethyl-1-thia-3-azaspiro[4.5]dec-3-ene 、 苯甲酰氯 在 copper(l) iodide 、 炔丙基脲 、 N,N-二异丙基乙胺 作用下, 以 二氯甲烷 为溶剂, 反应 1.0h, 以13%的产率得到(R/S)-3-benzoyl-4-benzoyloxy-2,2-dimethyl-1-thia-3-azaspiro[4.5]decane参考文献:名称:连续多组分反应:合成3-酰基-4-炔基取代的1,3-噻唑烷摘要:‡这些作者对这项工作做出了同等的贡献。 抽象的 这项工作描述了包含噻唑烷和炔丙基酰胺基序的化合物的合成。它们的制备遵循包含两个多组分反应的合成路线。首先,使用Asinger四组分反应制备3-噻唑啉和3-恶唑啉。其次,这些杂环亚胺通过使用酰氯和末端炔烃的铜催化三组分反应转化为炔丙基酰胺。合成途径的特征是温和的条件,并且可以容忍许多官能团。还提出了意外的α-炔氧基酰胺的形成。 这项工作描述了包含噻唑烷和炔丙基酰胺基序的化合物的合成。它们的制备遵循包含两个多组分反应的合成路线。首先,使用Asinger四组分反应制备3-噻唑啉和3-恶唑啉。其次,这些杂环亚胺通过使用酰氯和末端炔烃的铜催化三组分反应转化为炔丙基酰胺。合成途径的特征是温和的条件,并且可以容忍许多官能团。还提出了意外的α-炔氧基酰胺的形成。DOI:10.1055/s-0036-1591873
文献信息
-
Electrophilic Sulfonium-Promoted Peptide and Protein Amidation in Aqueous Media作者:Chuan Wan、Yuan Feng、Zhanfeng Hou、Chenshan Lian、Liang Zhang、Yuhao An、Jinming Sun、Dongyan Yang、Chenran Jiang、Feng Yin、Rui Wang、Zigang LiDOI:10.1021/acs.orglett.1c04017日期:2022.1.21A novel amidation strategy using electrophilic sulfonium, which is soluble and stable in aqueous conditions, was developed. The sulfoniums could activate thioacid and carboxyl acid to efficiently react with amines to afford amides. This method enables applications in amidation in both aqueous media and solid-phase peptide synthesis, peptide/protein modifications, and reactive lysines of a proteome
-
腈及其相应胺的制造方法申请人:中国石油化工股份有限公司公开号:CN104557610B公开(公告)日:2018-04-27本发明涉及一种腈的制造方法,与现有技术相比,具有氨源用量显著降低、环境压力小、能耗低、生产成本低、腈产物的纯度和收率高等特点,并且能够获得结构更为复杂的腈。本发明还涉及由该腈制造相应胺的方法。
-
Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death作者:Laura E Sanman、Yu Qian、Nicholas A Eisele、Tessie M Ng、Wouter A van der Linden、Denise M Monack、Eranthie Weerapana、Matthew BogyoDOI:10.7554/elife.13663日期:——
When innate immune cells such as macrophages are challenged with environmental stresses or infection by pathogens, they trigger the rapid assembly of multi-protein complexes called inflammasomes that are responsible for initiating pro-inflammatory responses and a form of cell death termed pyroptosis. We describe here the identification of an intracellular trigger of NLRP3-mediated inflammatory signaling, IL-1β production and pyroptosis in primed murine bone marrow-derived macrophages that is mediated by the disruption of glycolytic flux. This signal results from a drop of NADH levels and induction of mitochondrial ROS production and can be rescued by addition of products that restore NADH production. This signal is also important for host-cell response to the intracellular pathogen Salmonella typhimurium, which can disrupt metabolism by uptake of host-cell glucose. These results reveal an important inflammatory signaling network used by immune cells to sense metabolic dysfunction or infection by intracellular pathogens.
当巨噬细胞等先天性免疫细胞受到环境压力或病原体感染的挑战时,它们会触发被称为炎性体的多蛋白复合物的快速组装,这种复合物负责启动促炎反应和一种被称为脓毒症的细胞死亡形式。我们在本文中描述了一种细胞内触发 NLRP3 介导的炎症信号传导、IL-1β 生成和小鼠骨髓巨噬细胞热噬的机制,这种机制是由糖酵解通量的破坏介导的。这一信号是由 NADH 水平下降和诱导线粒体 ROS 生成引起的,加入能恢复 NADH 生成的产品后即可缓解。这种信号对于宿主细胞对胞内病原体鼠伤寒沙门氏菌的反应也很重要,鼠伤寒沙门氏菌可通过摄取宿主细胞的葡萄糖来破坏新陈代谢。这些结果揭示了免疫细胞用于感知代谢功能障碍或细胞内病原体感染的重要炎症信号网络。 -
Synthesis of peptide homo‐ and heterodimers as potential mimics of platelet‐derived growth factor BB作者:Louise A. Stubbing、Harveen Kaur、Sheryl X. Feng、Miranda Aalderink、Michael Dragunow、Margaret A. BrimbleDOI:10.1002/pep2.24150日期:2020.7disease. The platelet‐derived growth factor receptor β (PDGFRβ)/platelet‐derived growth factor BB (PDGF‐BB) signalling pathway is key to the regulation of pericyte survival and proliferation. A series of peptide dimers mimicking the ligand PDGF‐BB were prepared in the hope of stimulating PDGFRβ internalisation and activation of this pathway. Copper‐catalysed azide‐alkyne cycloaddition of peptide monomers
-
4R- and 4S-iodophenyl hydroxyproline, 4R-pentynoyl hydroxyproline, and S-propargyl-4-thiolphenylalanine: conformationally biased and tunable amino acids for bioorthogonal reactions作者:Christina R. Forbes、Anil K. Pandey、Himal K. Ganguly、Glenn P. A. Yap、Neal J. ZondloDOI:10.1039/c5ob02473k日期:——4-thiolphenylalanine was synthesized via copper-mediated cross-coupling reaction of thioacetic acid with protected 4-iodophenylalanine, followed by thiolysis and alkylation. This amino acid combines an alkyne functional group with an aromatic amino acid and the ability to tune aromatic and side chain properties via sulfur oxidation. These amino acids provide novel loci for peptide functionalization, with greater生物正交反应允许将新功能引入肽、蛋白质和其他生物分子中。用于生物正交反应的最容易获得的氨基酸具有适度的构象偏好或分子相互作用的基础。在此,我们描述了用于生物正交反应的 4 种含有官能团的新型氨基酸的合成。羟脯氨酸的( 2S , 4R )-和( 2S , 4S )-碘苯醚能够在水中通过快速、特异性的Suzuki和Sonogashira反应进行修饰。这些氨基酸(如 Boc-、Fmoc- 和游离氨基酸)的合成是通过简洁的序列实现的。这些氨基酸表现出明确的构象偏好,其中 4 S-碘苯基羟脯氨酸在晶体学上表现出 β 转角 ( phi , ψ ∼ –80°, 0°) 或相对延伸 ( phi , ψ ∼ –80°, +170°)构象,而 4 R -非对映异构体更喜欢更紧凑的构象 ( phi ∼ –60°)。芳氧基脯氨酸非对映体以高度不同的方式呈现芳基,表明它们在分子设计、药物化学和催化中的立体定向用途。因此,
表征谱图
-
氢谱1HNMR
-
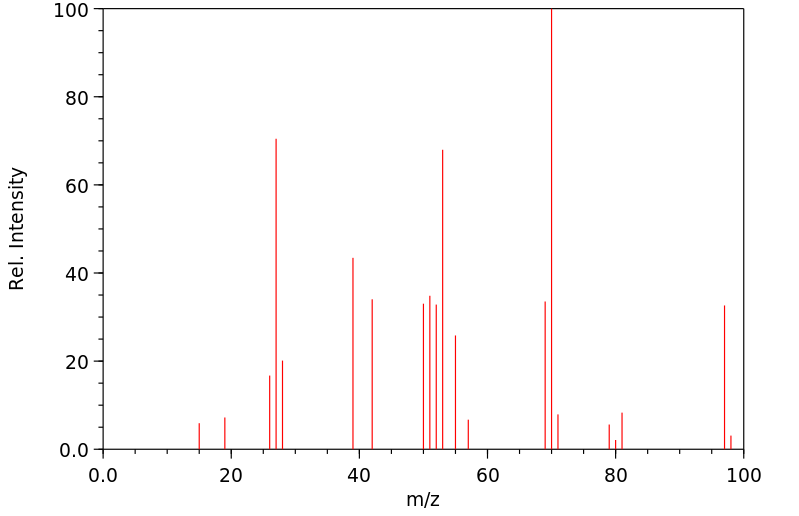
质谱MS
-
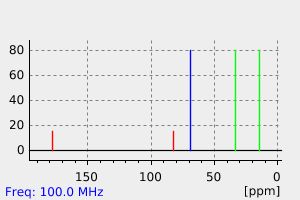
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息