西酞普兰 | 59729-33-8
中文名称
西酞普兰
中文别名
氰酞氟苯胺;1-(4-氟苯基)-1,3-二氢-5-异苯并呋喃腈氢溴酸盐;西酞普兰溴水合物;依地普仑;1-[3-(二甲氨基)丙基]-1-(4-氟苯基)-1,3-二氢-5-异苯并呋喃甲腈
英文名称
1-(3-dimethylamino-propyl)-1-(4-fluoro-phenyl)-1,3-dihydro-isobenzofuran-5-carbonitrile
英文别名
Citalopram;1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile;1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3H-2-benzofuran-5-carbonitrile
CAS
59729-33-8
化学式
C20H21FN2O
mdl
MFCD00865398
分子量
324.398
InChiKey
WSEQXVZVJXJVFP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:156-157 °C
-
沸点:bp0.03 175-181°
-
密度:1.18±0.1 g/cm3(Predicted)
-
溶解度:甲醇(微溶)、水(微溶)
-
物理描述:Solid
-
颜色/状态:Fine white to off-white powder
-
蒸汽压力:1.13X10-7 mm Hg at 25 °C (est)
-
水溶性:-4.7
-
稳定性/保质期:
Stable under recommended storage conditions. /Citalopram hydrobromide/
-
分解:When heated to decomposition it emits very toxic fumes of /nitrogen oxides, hydrogen fluoride and hydrogen bromides/
-
解离常数:pKa = 9.78 (est)
-
碰撞截面:180.1 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)]
-
保留指数:2393.4;2408.6;2406.5;2391.3
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:24
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.35
-
拓扑面积:36.3
-
氢给体数:0
-
氢受体数:4
ADMET
代谢
西酞普兰主要通过肝脏的N-去甲基化代谢为其主要代谢物,由CYP2C19和CYP3A4酶代谢为去甲基西酞普兰。其他代谢物包括通过CYP2D6代谢的二去甲基西酞普兰,以及通过单胺氧化酶和乙醛氧化酶代谢的西酞普兰N-氧化物。它是一种脱氨的丙酸衍生物。单次服用西酞普兰后,血药浓度峰值在大约4小时出现。这种药物在血浆中主要以西酞普兰的形式存在,没有发生变化。细胞色素P450(CYP)3A4和2C19同种酶在产生去甲基西酞普兰方面起着重要作用。去甲基西酞普兰似乎进一步通过CYP2D6的N-去甲基化代谢为二去甲基西酞普兰。与母药相比,西酞普兰代谢物在药理活性上很小,不太可能对西酞普兰的临床效果产生影响。
Citalopram is metabolized mainly in the liver via <i>N</i>-demethylation to its main metabolite, _demethylcitalopram_ by CYP2C19 and CYP3A4. Other metabolites include _didemethylcitalopram_ via CYP2D6 metabolism, and _citalopram <i>N</i>-oxide_ via monoamine oxidase enzymes and aldehyde oxidase. It is a deaminated propionic acid derivative. After a single dose of citalopram, peak blood concentrations occur at approximately 4 hours. This drug in is found mainly unchanged in the plasma as citalopram. Cytochrome P450 (CYP) 3A4 and 2C19 isozymes appear to be heavily involved in producing _demethylcitalopram_. Demethylcitalopram appears to be further <i>N</i>-demethylated by CYP2D6 to didemethylcitalopram. Citalopram metabolites exert little pharmacologic activity in comparison to the parent drug and are not likely to contribute to the clinical effect of citalopram.
来源:DrugBank
代谢
在2002年的一项研究中,11名孕妇在怀孕期间服用了西酞普兰(20-40毫克/天);其中10名孕妇在整个孕期服用,1名孕妇从孕20周开始服用。两种代谢物平均水平在怀孕期间比产后两个月显著升高,表明诱导了CYP2D6同工酶。正常新生儿中西酞普兰、去甲基西酞普兰和双去甲基西酞普兰的谷浓度分别为母体浓度的64%,66%和68%。
In a 2002 study, 11 women took citalopram (20-40 mg/day) during pregnancy; 10 throughtout gestation and 1 starting at 20 weeks' gestation. The mean ratio of two metabolites was significantly higher during pregnancy than at 2 months postdelivery, indicating induction of the CYP2D6 isoenzyme. The trough plasma concentration of citalopram, desmethylcitalopram, and didesmethylcitalopram in the normal new borns were 64%, 66%, and 68% of the maternal concentrations, respectively.
来源:Hazardous Substances Data Bank (HSDB)
代谢
选择性5-羟色胺再摄取抑制剂类抗抑郁药西酞普兰(CT),以标记形式14(C)-CT给药,作为50毫升水溶液的单次口服剂量(0.1毫摩尔/30微居里/1.1兆贝克)给四名健康男性志愿者。血液和血浆中的放射性浓度相似。...尿液成分的高效液相色谱(HPLC)分析显示,除了已知的西酞普兰代谢物外,还存在三种葡萄糖苷酸。7天内收集的尿液中CT及其代谢物的相对量为:CT(26%),N-去甲基-CT(DCT,19%),N,N-二去甲基-CT(DDCT,9%),N-氧化物(7%),CT的四价铵葡萄糖苷酸(CT-GLN,14%),DDCT的N-葡萄糖苷酸(DDCT-GLN,6%)以及由CT的N,N-二甲胺脱氨形成的酸代谢物的葡萄糖苷酸(CT-acid-GLN,12%)。通过制备色谱法分离出CT-GLN,并通过LC-MS-MS和核磁共振(NMR)进行鉴定。DDCT-GLN和CT-acid-GLN通过LC-MS鉴定。本研究表明,长期的肾排泄是主要的消除途径,只有一小部分随粪便排出。尿液中排出的剂量中,相当一部分是CT和DDCT的N-葡萄糖苷酸以及CT-acid的O-酰基葡萄糖苷酸。
The antidepressant citalopram (CT), a selective serotonin uptake inhibitor, was given in its labelled form, 14(C)-CT, as a single oral dose in 50 mL aqueous solution (0.1 mmol/30 uCi/1.1 MBq) to four healthy male volunteers. Concentrations of radioactivity in whole blood and plasma were similar. ... The HPLC profile of urinary components showed that besides the known metabolites of citalopram, three glucuronides were present. The relative amounts of CT and its metabolites in urine collected for 7 days were: CT (26 %), N-demethyl-CT (DCT, 19%), N,N-didemethyl-CT (DDCT,9%), the N-oxide (7%), the quaternary ammonium glucuronide of CT (CT-GLN, 14%), the N-glucuronide of DDCT (DDCT-GLN, 6%), and the glucuronide of the acid metabolite (CT-acid-GLN, 12%) formed by N,N-dimethyl deamination of CT. CT-GLN was isolated using preparative chromatography and identified by LC-MS-MS and NMR. DDCT-GLN and CT-acid-GLN were identified by LC-MS. This study shows that protracted renal excretion represents the major route of elimination, with a small fraction voided with feces. A considerable portion of the urinary excreted dose consists of N-glucuronides of CT and DDCT together with the O-acyl glucuronide of CT-acid.
来源:Hazardous Substances Data Bank (HSDB)
代谢
这项研究旨在确定最终催化西酞普兰局部脑代谢的酶系统。... 在大鼠脑微粒体和人大鼠脑线粒体中研究了西酞普兰及其对映体和脱甲基代谢物的代谢。在大鼠脑中没有观察到细胞色素P-450介导的转化。... 在大鼠全脑和人的前额叶皮质、壳核、小脑及五个脑的白质中,通过立体选择性测量西酞普兰丙酸的产生来确定单胺氧化酶的活性。所有底物都被两种形式的单胺氧化酶代谢,除了在大鼠脑中,无法检测到单胺氧化酶B的活性。在人前额叶皮质中西酞普兰生物转化的表观Km和Vmax分别为266 uM和6.0 pmol/min/mg蛋白质,而单胺氧化酶A分别为856 uM和6.4 pmol/min/mg蛋白质。这些Km值与单胺氧化酶对血清素和多巴胺代谢的Km值相同。...
This study was conducted to identify enzyme systems eventually catalyzing a local cerebral metab of citalopram. ... The metab of citalopram, of its enantiomers and demethylated metabolites was investigated in rat brain microsomes & in rat and human brain mitochondria. No cytochrome P-450 mediated transformation was observed in rat brain. ... In rat whole brain and in human frontal cortex, putamen, cerebellum & white matter of five brains monoamine oxidase activity was determined by the stereoselective measurement of the production of citalopram propionate. All substrates were metabolized by both forms of MAO, except in rat brain, where monoamine oxidase B activity could not be detected. Apparent Km and Vmax of S-citalopram biotransformation in human frontal cortex by monoamine oxidase B were found to be 266 uM & 6.0 pmol min/mg protein & by monoamine oxidase A 856 uM & 6.4 pmol min/mg protein, respectively. These Km values are in the same range as those for serotonin & dopamine metab by monoamine oxidases. ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
西酞普兰...部分通过CYP2C19和部分通过CYP3A4脱甲基转化为N-去甲基西酞普兰,而N-去甲基西酞普兰进一步通过CYP2D6脱甲基转化为同样无活性的代谢物二去甲基西酞普兰。这两种代谢物均无活性。...在体外,西酞普兰不抑制CYP或仅轻微抑制。在健康受试者和患者中进行的一系列研究已经证实,这一点在体内也成立。因此,当西酞普兰与CYP1A2底物(氯氮平和茶碱)、CYP2C9(华法林)、CYP2C19(丙咪嗪和美芬妥因)、CYP2D6(麻黄碱、丙咪嗪和阿米替林)以及CYP3A4(卡马西平和三唑仑)一起给药时,没有观察到药代动力学的变化或只有非常小的变化。...
Citalopram ... is N-demethylated to N-desmethylcitalopram partially by CYP2C19 & partially by CYP3A4 & N-desmethylcitalopram is further N-demethylated by CYP2D6 to the likewise inactive metabolite di-desmethylcitalopram. The two metabolites are not active. ... In vitro citalopram does not inhibit CYP or does so only very moderately. A number of studies in healthy subjects and patients have confirmed, that this also holds true in vivo. Thus no change in pharmacokinetics or only very small changes were observed when citalopram was given with CYP1A2 substrates (clozapine & therophylline), CYP2C9 (warfarin), CYP2C19 (imipramine & mephenytoin), CYP2D6 (sparteine, imipramine & amitriptyline) and CYP3A4 (carbamazepine & triazolam). ...
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和用途:西酞普兰是一种固体。它是一种血清素再摄取抑制剂,第二代抗抑郁药。西酞普兰片剂用于治疗抑郁症。
人类研究:西酞普兰过量(单独或与其他药物和/或酒精联合使用)最常见的症状包括头晕、出汗、恶心、呕吐、震颤、嗜睡和窦性心动过速。在更罕见的情况下,观察到的症状包括遗忘、混乱、昏迷、惊厥、过度换气、发绀、横纹肌溶解和心电图变化(包括QTc延长、结性心律、室性心律失常,以及非常罕见的尖端扭转型室速)。急性肾衰竭在西酞普兰过量时非常罕见地被报道。在子宫内接触西酞普兰(30毫克/天)、帕罗西汀(10-40毫克/天)或氟西汀(20毫克/天)的五名男婴在出生时或出生后几天内出现戒断症状,持续长达1个月。症状包括易怒、持续哭泣、颤抖、肌张力增加、饮食和睡眠问题以及惊厥。晚期妊娠接触西酞普兰可能与新生儿毒性综合症有关,该综合症在出生时或出生后不久立即发作,有时可能被误认为是新生儿戒断综合症。一名3860克的婴儿在40周妊娠时分娩。母亲在分娩当天一直服用西酞普兰20毫克/天。西酞普兰在体外人淋巴细胞染色体畸变分析中不具有裂变性。动物研究:西酞普兰通过饮食给予小鼠和大鼠,分别为18个月和24个月。没有证据表明西酞普兰在小鼠接受高达240毫克/千克/天的剂量时具有致癌性。在大鼠接受8或24毫克/千克/天剂量的情况下,小肠腺癌的发生率增加。这些发现对人类的相关性尚不清楚。在对西酞普兰进行的2年致癌性研究中,观察到白化大鼠视网膜的病理变化(变性/萎缩)。在一项兔研究中,在高达16毫克/千克/天的剂量下,没有观察到对胚胎/胎儿发育的负面影响。当雌性大鼠在晚期妊娠至断奶期间接受西酞普兰(4.8、12.8或32毫克/千克/天)治疗时,在出生后前4天内观察到后代死亡率增加和持续的后代生长迟缓。在两项大鼠胚胎/胎儿发育研究中,在器官形成期给怀孕动物口服西酞普兰(32、56或112毫克/千克/天)导致在高剂量下胚胎/胎儿生长和存活率降低,胎儿异常(包括心血管和骨骼缺陷)的发生率增加。西酞普兰在体外细菌反向突变分析(Ames试验)中在5种细菌株中的2种(Salmonella TA98和TA1537)在无代谢激活的情况下具有诱变性。它在体外中国仓鼠肺细胞染色体畸变分析中,在存在和不存在代谢激活的情况下具有裂变性。西酞普兰在体外小鼠淋巴瘤细胞哺乳动物正向基因突变分析(HPRT)或在大鼠肝脏的耦合体外/体内非计划DNA合成(UDS)分析中不具有诱变性。
IDENTIFICATION AND USE: Citalopram is a solid. It is a serotonin uptake inhibitor, and second-generation antidepressive agent. Citalopram tablets are indicated for the treatment of depression. HUMAN STUDIES: Symptoms most often accompanying citalopram overdose, alone or in combination with other drugs and/or alcohol, included dizziness, sweating, nausea, vomiting, tremor, somnolence, and sinus tachycardia. In more rare cases, observed symptoms included amnesia, confusion, coma, convulsions, hyperventilation, cyanosis, rhabdomyolysis, and ECG changes (including QTc prolongation, nodal rhythm, ventricular arrhythmia, and very rare cases of torsade de pointes). Acute renal failure has been very rarely reported accompanying overdose. Five male infants exposed to citalopram (30 mg/day), paroxetine (10-40 mg/day) or fluoxetine (20 mg/day) during gestation exhibited withdrawal symptoms at or within a few days of birth and lasting up to 1 month. Symptoms included irritability, constant crying, shivering, increased tonus, eating and sleeping problems, and convulsions. Late gestational exposure to citalopram, may be associated with a neonatal toxicity syndrome with immediate onset at birth or soon after birth and sometimes may be mistaken for neonatal withdrawal syndrome. A 3860 g infant was delivered at 40 weeks gestation. The mother had been taking citalopram 20 mg/day until the day of delivery. Citalopram was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes. ANIMAL STUDIES: Citalopram was administered in the diet to mice and rats for 18 and 24 months, respectively. There was no evidence for carcinogenicity of citalopram in mice receiving up to 240 mg/kg/day. There was an increased incidence of small intestine carcinoma in rats receiving 8 or 24 mg/kg/day doses. The relevance of these findings to humans is unknown. Pathologic changes (degeneration/atrophy) were observed in the retinas of albino rats in the 2-year carcinogenicity study with citalopram. In a rabbit study, no adverse effects on embryo/fetal development were observed at doses of up to 16 mg/kg/day. When female rats were treated with citalopram (4.8, 12.8, or 32 mg/kg/day) from late gestation through weaning, increased offspring mortality during the first 4 days after birth and persistent offspring growth retardation were observed at the highest dose. In two rat embryo/fetal development studies, oral administration of citalopram (32, 56, or 112 mg/kg/day) to pregnant animals during the period of organogenesis resulted in decreased embryo/fetal growth and survival and an increased incidence of fetal abnormalities (including cardiovascular and skeletal defects) at the high dose. Citalopram was mutagenic in the in vitro bacterial reverse mutation assay (Ames test) in 2 of 5 bacterial strains (Salmonella TA98 and TA1537) in the absence of metabolic activation. It was clastogenic in the in vitro Chinese hamster lung cell assay for chromosomal aberrations in the presence and absence of metabolic activation. Citalopram was not mutagenic in the in vitro mammalian forward gene mutation assay (HPRT) in mouse lymphoma cells or in a coupled in vitro/in vivo unscheduled DNA synthesis (UDS) assay in rat liver.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
这种抗抑郁、抗强迫症和抗暴食行为的药物——西酞普兰的作用被认为与其抑制中枢神经系统神经元对5-羟色胺(血清素)的摄取有关。西酞普兰阻断了神经元膜上5-羟色胺再摄取泵的功能,增强了5-羟色胺在5HT1A自受体的作用。与三环类抗抑郁药物相比,选择性5-羟色胺再摄取抑制剂(SSRIs)与组胺、乙酰胆碱和去甲肾上腺素受体的亲和力显著较低。
The antidepressant, antiobsessive-compulsive, and antibulimic actions of citalopram are presumed to be linked to its inhibition of CNS neuronal uptake of serotonin. Citalopram blocks the reuptake of serotonin at the serotonin reuptake pump of the neuronal membrane, enhancing the actions of serotonin on 5HT<sub>1A</sub> autoreceptors. SSRIs bind with significantly less affinity to histamine, acetylcholine, and norepinephrine receptors than tricyclic antidepressant drugs.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
DILI 注解:较少的药物性肝损伤关注
DILI Annotation:Less-DILI-Concern
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
严重程度等级:7
Severity Grade:7
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
吸收、分配和排泄
从胃肠道快速且良好吸收。单次口服给药后,血浆峰浓度在4小时内出现。口服给药后的生物利用度为80%。食物不影响吸收。
Rapidly and well absorbed from the GI tract. Peak plasma concentrations occur within 4 hours of a single orally administered dose. Bioavailability is 80% following oral administration. Food does not affect absorption.
来源:DrugBank
吸收、分配和排泄
12-23%的西酞普兰口服剂量在尿液中以未改变的形式被找到,而10%的剂量在粪便中被找到。
12-23% of an oral dose of citalopram is found unchanged in the urine, while 10% of the dose is found in the faeces.
来源:DrugBank
吸收、分配和排泄
12 L/kg Citalopram is highly lipophilic and likely widely distributed throughout the body, including the blood-brain-barrier. However, its metabolite, _demethylcitalopram_ does not penetrate the blood-brain-barrier well.
来源:DrugBank
吸收、分配和排泄
西酞普兰的系统清除率为330 mL/min,其中大约20%通过肾脏清除。
The systemic clearance of citalopram is 330 mL/min, with approximately 20% renal clearance.
来源:DrugBank
吸收、分配和排泄
像其他选择性5-羟色胺再摄取抑制剂一样,西酞普兰是一种高度亲脂性的化合物,口服给药后似乎能从胃肠道快速且充分地吸收。生产厂家指出,在单次口服40毫克西酞普兰片剂后,平均峰浓度约为44纳克/毫升,在大约4小时出现。
Like other selective serotonin-reuptake inhibitors, citalopram is a highly lipophilic compound that appears to be rapidly and well absorbed from the GI tract following oral administration. Following a single 40-mg oral dose of citalopram as a tablet, the manufacturer states that peak plasma concentrations averaging approximately 44 ng/mL occur at about 4 hours.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:6.1(b)
-
危险品运输编号:3249
-
包装等级:III
-
危险类别:6.1(b)
-
储存条件:.Store at room temperature.
SDS
制备方法与用途
西酞普兰(Citalopram)概述
西酞普兰(Citalopram),是选择性五羟色胺回收抑制剂的一种。抑郁症作为精神科常见的精神疾病,其患者常伴有焦虑症状。这种抑郁与焦虑的共病现象在年轻和老年患者中尤为常见,甚至成为患者的就医原因之一。
西酞普兰治疗伴随焦虑症状的老年抑郁症患者效果显著,因其作用温和、药物相互作用较少及安全性好,在老年人群中的应用较为广泛。此外,它对其他神经递质的影响较小,特别适用于老年人。西酞普兰是首个在丹麦获准用于临床的五羟色胺再摄取抑制剂,并于1998年在美国成功上市。
药理作用西酞普兰通过选择性地抑制突触前膜对五羟色胺(5-HT)的再吸收,增加脑内5-HT水平,从而发挥抗抑郁效果。它主要用于治疗抑郁症、老年性痴呆及多发梗死性痴呆等。
化学性质与用途西酞普兰为油状物,沸点175~181℃/4.00Pa;其氢溴酸盐(Citalopram Hydmbmmide)熔点为182--183℃,从异丙醇结晶而成;草酸盐(Citalopram Oxalate)熔点为164~166℃。
生产方法 氢溴酸西酞普兰的合成在通氮气、温度控制在60-70℃下,将21g 50%氢化钠(溶于矿物油中)溶于90ml二甲亚砜中制得甲亚磺酰甲基钠溶液。冷却条件下滴入96g的1-(4-氟苯基)-1-二氢-S-异苯并呋喃腈(溶于150ml二甲亚砜),保持反应液温度为25℃,维持10min。随后快速加入53g的3-二甲氨基丙基氯(溶于25ml二亚砜中)加热至40℃,继续搅拌50min。
将上述混合物倾入冰水中进行萃取后,乙醚提取液再用20%乙酸水溶液处理。随后碱化乙酸水溶液并再次使用乙醚进行萃取。最终经无水碳酸钾干燥、真空浓缩等步骤制得西酞普兰。
药理作用与临床应用- 治疗抑郁症:适用于各种类型的抑郁症。
- 老年性痴呆及多发梗死性痴呆的治疗,尤其适用于轻度至中度症状患者。
- 成人剂量:每日一次片剂(20mg),必要时可增至40mg或60mg。
- 老年患者剂量减半,建议每日0.5~1.5片(10~30mg)。
- 治疗时间通常需要数月。
- 慎用人群:心肌梗死、单胺氧化酶抑制剂联用者等应慎用西酞普兰。
- 药物相互作用:禁止与单胺氧化酶抑制剂联合使用,停药后至少两周方可换药。
- 自杀风险:治疗期间可能持续存在自杀倾向,躁狂期应及时停药并给予适当的精神抑制治疗。
- 低钠血症及抗利尿激素分泌异常综合征的监测至关重要。
- 避免操作机械和驾驶车辆,同时禁用于饮酒。
- 孕妇及哺乳期妇女使用需谨慎,FDA分类为C级。
- 儿童用药的安全性和有效性尚未确定。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(4-氟苯基)-1,3-二氢-1-[3-(甲基氨基)丙基]异苯并呋喃-5-甲腈 rac-desmethylcitalopram 62498-67-3 C19H19FN2O 310.371 1-(3-氨基丙基)-1-(4-氟苯基)-1,3-二氢-5-异苯并呋喃甲腈 didesmethylcitalopram 62498-69-5 C18H17FN2O 296.344 —— 1-(4-fluorophenyl)-1-(3-oxopropyl)-1,3-dihydroisobenzofuran-5-carbonitrile 211800-31-6 C18H14FNO2 295.313 —— 3-(5-(aminomethyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 125803-03-4 C20H25FN2O 328.43 西酞普兰羧酸杂质 1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxylic acid 440121-09-5 C20H22FNO3 343.398 —— 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran —— C19H22FNO 299.388 1-[3-(二甲基氨基)丙基]-1-(4-氟苯基)-1,3-二氢-5-异苯并呋喃甲酰胺 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxamide 64372-56-1 C20H23FN2O2 342.413 1-(4-氟苯基)-1-(3-二甲基氨基丙基)-5-氯二氢异苯并呋喃 Lu 10-134-C 64169-45-5 C19H21ClFNO 333.833 4-(4-二甲胺基-1-对氟苯基-1-羟基丁基)-3-(羟甲基)苯腈 4-[4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile 103146-25-4 C20H23FN2O2 342.413 (-)4[4-(二甲胺基)-1-(4-氟苯基)-1-(羟基丁基)-3-(羟基 甲基)氰苯.氢溴酸盐 (-)-(S)-4-(4-dimethylamino-1-(4'-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)-benzonitrile 488787-59-3 C20H23FN2O2 342.413 —— 3-(1-(4-fluorophenyl)-5-iodo-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine —— C19H21FINO 425.285 3-(5-溴-1-(4-氟苯基)-1,3-二氢异苯并呋喃-1-基)-N,N-二甲基丙烷-1-胺 {3-[5-bromo-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-propyl}-dimethylamine 64169-39-7 C19H21BrFNO 378.284 —— 5-bromo-1-(4-fluorophenyl)-1-(3-methylaminopropyl)phthalane 945543-92-0 C18H19BrFNO 364.257 —— 5-Amino-1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran —— C19H23FN2O 314.403 —— 1-(4-fluorophenyl)-1-[(3-p-toluenesulfonyloxy)propyl]-1,3-dihydro-5-isobenzo-furancarbonitrile 345663-50-5 C25H22FNO4S 451.518 —— (±)-1-(4-fluorophenyl)-1-formyl-1,3-dihydroisobenzofuran-5-carbonitrile —— C16H10FNO2 267.259 —— 4,4-dimethyl-[2-[3-hydroxymethyl-4-[4-fluoro-a-hydroxy-a-(dimethylamino)propyl]benzyl]phenyl]oxazoline 265137-38-0 C24H31FN2O3 414.52 1-(4-氟苯基)-1,3-二氢异苯并呋喃-5-腈 1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 64169-67-1 C15H10FNO 239.249 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (R)-西酞普兰 (S)-1-[3-(dimethylamino)propyl]-1-(4-flouorphenyl)-1,3-dihydro-5-isobenzofuran carbonitrile 128196-02-1 C20H21FN2O 324.398 依他普仑 escitalopram 128196-01-0 C20H21FN2O 324.398 1-(4-氟苯基)-1,3-二氢-1-[3-(甲基氨基)丙基]异苯并呋喃-5-甲腈 rac-desmethylcitalopram 62498-67-3 C19H19FN2O 310.371 —— (-)-Desmethylcitalopram —— C19H19FN2O 310.371 —— 1-(4-fluorophenyl)-1-(3-(methyl(4-phenylbutyl)amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-91-3 C29H31FN2O 442.576 —— 1-(3-(butyl(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-89-9 C23H27FN2O 366.479 —— 1-(4-fluorophenyl)-1-(3-(methyl(propyl)amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-88-8 C22H25FN2O 352.452 —— 1-(3-((2-aminoethyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-16-5 C21H24FN3O 353.439 —— 1-(3-((cyclopropylmethyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-87-7 C23H25FN2O 364.463 艾司西酞普兰 E Citalopram N-Oxide 63284-72-0 C20H21FN2O2 340.397 —— 1-(4-fluorophenyl)-1-(3-(methyl(phenethyl)amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-90-2 C27H27FN2O 414.523 —— 1-(4-fluorophenyl)-1-(3-(methyl(pyridin-4-yl)amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-96-8 C25H24FN3O 401.483 —— 3-(5-(aminomethyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 125803-03-4 C20H25FN2O 328.43 西酞普兰甲醛 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbaldehyde 227954-87-2 C20H22FNO2 327.399 —— 1-(4-fluorophenyl)-1-(3-(methyl(4-oxocyclohexyl)amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-21-2 C25H27FN2O2 406.5 —— 1-(3-(((1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-yl)methyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-05-2 C39H41F2N3O2 621.77 —— 1-(4-fluorophenyl)-1-(3-(methyl(naphthalen-2-ylmethyl)amino)-propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-01-8 C30H27FN2O 450.556 —— 3-(5-((((1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-yl)methyl)amino)methyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-72-0 C40H47F2N3O2 639.829 西酞普兰羧酸杂质 1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxylic acid 440121-09-5 C20H22FNO3 343.398 —— 1-(3-((4-(dimethylamino)benzyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-94-6 C28H30FN3O 443.564 —— methyl 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxylate 1507401-62-8 C21H24FNO3 357.425 —— 3-(1-(4-fluorophenyl)-5-(piperidin-1-ylmethyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-63-9 C25H33FN2O 396.548 1-[3-(二甲基氨基)丙基]-1-(4-氟苯基)-1,3-二氢-5-异苯并呋喃甲酰胺 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxamide 64372-56-1 C20H23FN2O2 342.413 —— 3-(1-(4-fluorophenyl)-5-(morpholinomethyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-64-0 C24H31FN2O2 398.521 —— 3-(1-(4-fluorophenyl)-5-(((naphthalen-2-ylmethyl)amino)-methyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-70-8 C31H33FN2O 468.614 —— 1-(4-fluorophenyl)-1-(3-(methyl(1,4-dioxaspiro[4.5]decan-8-yl)-amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-18-7 C27H31FN2O3 450.553 1-[3-(二甲基氨基)丙基]-1-(4-氟苯基)-1,3-二氢-3-氧代-5-异苯并呋喃甲腈 3-oxocitalopram 372941-54-3 C20H19FN2O2 338.381 —— N'-benzoyl-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzo furan-5-carbohydrazide —— C27H28FN3O3 461.536 —— N'-(4-methylbenzoyl)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbohydrazide —— C28H30FN3O3 475.563 —— 1-(3-(((9H-fluoren-2-yl)methyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-03-0 C33H29FN2O 488.604 —— 1-(4-fluorophenyl)-1-(3-(methyl(quinolin-4-ylmethyl)amino)-propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-98-0 C29H26FN3O 451.543 —— 1-(3-(((1H-indol-3-yl)methyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-09-6 C28H26FN3O 439.532 —— 3-(1-(4-fluorophenyl)-5-((4-phenylpiperazin-1-yl)methyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-65-1 C30H36FN3O 473.634 —— N'-(2-iodobenzoyl)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro isobenzofuran-5-carbohydrazide —— C27H27FIN3O3 587.433 —— 1-(3-((benzo[b]thiophen-3-yl-methyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-93-5 C28H25FN2OS 456.584 —— N'-(2-bromobenzoyl)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbohydrazide —— C27H27BrFN3O3 540.432 —— 1-(3-((benzofuran-3-yl-methyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507401-92-4 C28H25FN2O2 440.517 —— 3-(5-((((9H-fluoren-2-yl)methyl)amino)methyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-71-9 C34H35FN2O 506.663 —— 1-(3-((2-(1,3-dioxoisoindolin-2-yl)ethyl)(methyl)amino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-14-3 C29H26FN3O3 483.542 —— N'-(2-methoxybenzoyl)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbohydrazide —— C28H30FN3O4 491.562 —— 3-(5-(((benzofuran-3-ylmethyl)amino)methyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-68-4 C29H31FN2O2 458.576 —— N'-(4-hydroxy-3-methoxybenzoyl)-1-[3-(dimethylamino)propyl]-1-(4-fluoro phenyl)-1,3-dihydroisobenzofuran-5-carbohydrazide —— C28H30FN3O5 507.562 —— 1-(4-fluorophenyl)-1-(3-(((5-methoxy-1H-indol-3-yl)methyl)-(methyl)amino)propyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-13-2 C29H28FN3O2 469.559 —— 3-(5-((4-(2,3-dichlorophenyl)piperazin-1-yl)methyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-67-3 C30H34Cl2FN3O 542.524 —— 3-(1-(4-fluorophenyl)-5-((4-(2-methoxyphenyl)piperazin-1-yl)-methyl)-1,3-dihydroisobenzofuran-1-yl)-N,N-dimethylpropan-1-amine 1507401-66-2 C31H38FN3O2 503.66 —— 1-(3-((4-(1H-indol-3-yl)cyclohex-3-en-1-yl)(methyl)amino)-propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile 1507402-22-3 C33H32FN3O 505.635 —— tert-butyl-3-(((3-(5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)propyl)(methyl)amino)methyl)-1H-indole-1-carboxylate 1507402-07-4 C33H34FN3O3 539.65 —— tert-butyl-3-(((3-(5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)propyl)(methyl)amino)methyl)-5-methoxy-1H-indole-1-carboxylate 1507402-11-0 C34H36FN3O4 569.676 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:以乙腈为氰源的镍催化(杂)芳基卤化物的氰化反应摘要:我们提出了一种高效的方法来氰化具有挑战性的基材,特别关注芳基氟化物。这种创新方法已成功扩展到涵盖多种芳基卤化物,强调了其多功能性和广泛的适用性。镍催化方案在温和的温度条件下利用乙腈,为氰化提供了清洁、安全的替代方案。值得注意的是,它采用无害、非气态、不含金属的氰化物源,并具有广泛的底物范围,可容纳芳基氯、氟化物、溴化物和碘化物。该反应对于乙腈特别有效。这种催化氰化过程是合成来曲唑、西酞普兰和其他 NNRTI 药物等药物的宝贵途径。从机理上讲,我们认为涉及零价镍和二价镍的催化循环对于该反应更为合理。DOI:10.1021/acscatal.3c05836
-
作为产物:描述:参考文献:名称:Structure−Activity Relationships for a Novel Series of Citalopram (1-(3-(Dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile) Analogues at Monoamine Transporters摘要:(+/−)-Citalopram(1, 1-(3-(二甲基氨基)丙基)-1-(4-氟苯基)-1,3-二氢异苯并呋喃-5-腈),及其eutomer(纯对映体),艾司西酞普兰(S-(+)-1)是选择性5-羟色胺再摄取抑制剂(SSRIs),临床上用于治疗焦虑和抑郁。为了进一步探索5-羟色胺转运体(SERT)处的构效关系,设计、合成了(+/-)-4-和5-取代的西酞普兰类似物,并在啮齿类动物的天然组织中评估了它们对SERT、多巴胺转运体(DAT)和去甲肾上腺素转运体(NET)的结合能力。许多这些类似物显示出高SERT结合亲和力(K-i = 1-40 nM),并且对NET和DAT具有选择性。合成了一些对映体类似物,其中S-和R-对映体在SERT处均保持了对映选择性,且S > R。此外,1和5的对映体对在同源的细菌亮氨酸转运体(LeuT)上的结合能力也被测试,结果显示低亲和力和缺乏对映选择性,这表明这些化合物在SERT上的结合位点与LeuT相比是独特的。这些新型配体将为阐明药物与蛋白质在SERT处的相互作用提供分子工具,并将这些作用与体内行为反应相关联。DOI:10.1021/jm1005034
-
作为试剂:描述:1-(4-氟苯基)-1,3-二氢异苯并呋喃-5-腈 、 N,N-二甲基-3-氯丙胺 在 ice 、 乙醚 、 溶剂黄146 、 sodium hydroxide 、 西酞普兰 作用下, 生成 西酞普兰参考文献:名称:Process for the isolation of high purity crystalline citalopram base摘要:提供了一种方法,用于从5-氰基邻苯二甲酸酯和N,N-二甲基氨丙基氯化物的烷基化反应混合物中,使用强碱在极性无水溶剂中直接分离高纯度结晶性西酞普兰(1-[3-二甲基氨基)丙基}-1-(4-氟苯基)-1,3-二氢-5-异苯并呋喃碳腈)碱。该方法包括:(a)用冰冷水稀释反应混合物,并用不溶于水的有机溶剂提取所得混合物;(b)再次用水不溶性有机溶剂提取酸性水溶液;(c)将酸性水溶液提取物与体积基于酸性水溶液中的水的量的相当的水亲和性有机溶剂稀释;(d)使用无机碱将pH调节到碱性,以沉淀出自由结晶碱;(e)通过过滤进一步分离沉淀的自由结晶碱。公开号:US07255741B2
文献信息
-
[EN] SUBSTITUTED N-HETEROCYCLIC CARBOXAMIDES AS ACID CERAMIDASE INHIBITORS AND THEIR USE AS MEDICAMENTS<br/>[FR] CARBOXAMIDES N-HÉTÉROCYCLIQUES SUBSTITUÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA CÉRAMIDASE ACIDE ET LEUR UTILISATION EN TANT QUE MÉDICAMENTS申请人:BIAL BIOTECH INVEST INC公开号:WO2021055627A1公开(公告)日:2021-03-25The invention provides substituted N-heterocyclic carboxamides and related compounds, compositions containing such compounds, medical kits, and methods for using such compounds and compositions to treat a medical disorder, e.g., cancer, lysosomal storage disorder, neurodegenerative disorder, inflammatory disorder, in a patient.这项发明提供了替代的N-杂环羧酰胺和相关化合物,含有这些化合物的组合物,医疗工具包,以及使用这些化合物和组合物治疗患者的医疗疾病(例如癌症、溶酶体贮积症、神经退行性疾病、炎症性疾病)的方法。
-
[EN] COMPOUNDS AND THEIR USE AS BACE INHIBITORS<br/>[FR] COMPOSÉS ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE BACE申请人:ASTRAZENECA AB公开号:WO2016055858A1公开(公告)日:2016-04-14The present application relates to compounds of formula (I), (la), or (lb) and their pharmaceutical compositions/preparations. This application further relates to methods of treating or preventing Αβ-related pathologies such as Down's syndrome, β- amyloid angiopathy such as but not limited to cerebral amyloid angiopathy or hereditary cerebral hemorrhage, disorders associated with cognitive impairment such as but not limited to MCI ("mild cognitive impairment"), Alzheimer's disease, memory loss, attention deficit symptoms associated with Alzheimer's disease, neurodegeneration associated with diseases such as Alzheimer's disease or dementia, including dementia of mixed vascular and degenerative origin, pre-senile dementia, senile dementia and dementia associated with Parkinson's disease.本申请涉及式(I)、(Ia)或(Ib)的化合物及其药物组合物/制剂。本申请进一步涉及治疗或预防与Αβ相关的病理学,如唐氏综合症,β-淀粉样蛋白血管病,如但不限于脑淀粉样蛋白血管病或遗传性脑出血,与认知损害相关的疾病,如但不限于MCI(“轻度认知损害”),阿尔茨海默病,记忆丧失,与阿尔茨海默病相关的注意力缺陷症状,与疾病如阿尔茨海默病或痴呆症相关的神经退行性疾病,包括混合性血管性和退行性起源的痴呆,早老性痴呆,老年性痴呆和与帕金森病相关的痴呆的方法。
-
New Drug Delivery System for Crossing the Blood Brain Barrier申请人:Lipshutz H. Bruce公开号:US20070203080A1公开(公告)日:2007-08-30New ubiquinol analogs are disclosed, as well as methods of using these compounds to deliver drug moieties to the body.新的泛醌类似物被披露,以及利用这些化合物将药物基团输送到人体的方法。
-
[EN] METHYL OXAZOLE OREXIN RECEPTOR ANTAGONISTS<br/>[FR] MÉTHYLOXAZOLES ANTAGONISTES DU RÉCEPTEUR DE L'OREXINE申请人:MERCK SHARP & DOHME公开号:WO2016089721A1公开(公告)日:2016-06-09The present invention is directed to methyl oxazole compounds which are antagonists of orexin receptors. The present invention is also directed to uses of the compounds described herein in the potential treatment or prevention of neurological and psychiatric disorders and diseases in which orexin receptors are involved. The present invention is also directed to compositions comprising these compounds. The present invention is also directed to uses of these compositions in the potential prevention or treatment of such diseases in which orexin receptors are involved.本发明涉及甲基噁唑化合物,其为促进睡眠的受体拮抗剂。本发明还涉及所述化合物在潜在治疗或预防涉及促进睡眠的神经和精神疾病和疾病中的用途。本发明还涉及包含这些化合物的组合物。本发明还涉及这些组合物在潜在预防或治疗涉及促进睡眠的疾病中的用途。
-
NAPHTHALENE-BASED INHIBITORS OF ANTI-APOPTOTIC PROTEINS申请人:Pellecchia Maurizio公开号:US20090105319A1公开(公告)日:2009-04-23Methods of using apogossypol and its derivatives for treating inflammation is disclosed. Also, there is described a group of compounds having structure A, or a pharmaceutically acceptable salt, hydrate, N-oxide, or solvate thereof are provided: wherein each R is independently selected from the group consisting of H, C(O)X, C(O)NHX, NH(CO)X, SO 2 NHX, and NHSO 2 X, wherein X is selected from the group consisting of an alkyl, a substituted alkyl, an aryl, a substituted aryl, an alkylaryl, and a heterocycle. Compounds of group A may be used for treating various diseases or disorders, such as cancer.
表征谱图
-
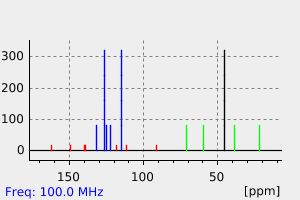
氢谱1HNMR
-
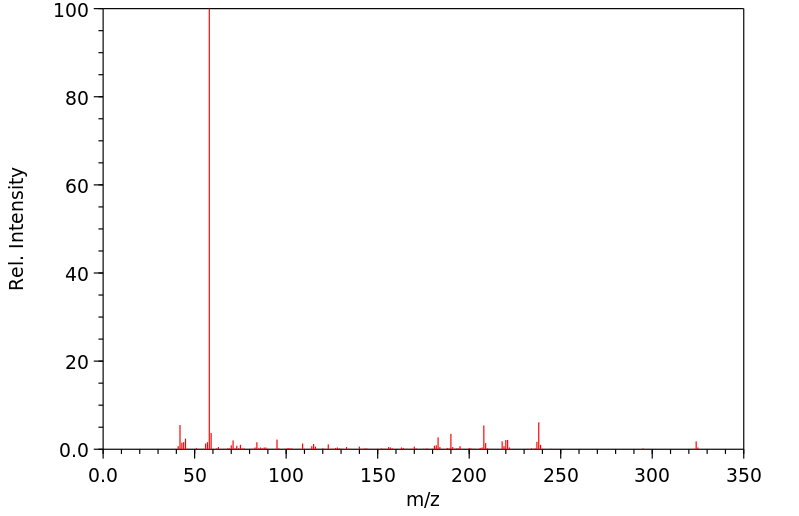
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫