乙二醇 | 107-21-1
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-13 °C (lit.)
-
沸点:195-198 °C
-
密度:1.113 g/mL at 25 °C (lit.)
-
蒸气密度:2.1 (vs air)
-
闪点:230 °F
-
溶解度:水:混溶
-
最大波长(λmax):λ: 260 nm Amax: ≤0.03λ: 280 nm Amax: ≤0.01
-
介电常数:37.0(20℃)
-
暴露限值:Ceiling limit in air for vapor and mist 50 ppm (~125 mg/m3) (ACGIH); TWA 10 mg/m3 (particulates) (MSHA).
-
LogP:-1.36 at 25℃
-
物理描述:Clear, colorless, syrupy, odorless liquid. [antifreeze] [Note: A solid below 9°F.]
-
颜色/状态:Clear, colorless, syrupy, liquid [Note: A solid below 9 degrees F].
-
气味:Odorless
-
味道:Sweet taste
-
蒸汽密度:2.14 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.092 mm Hg at 25 °C /Extrapolated/
-
亨利常数:Henry's Law constant = 6.00X10-8 atm-cu m/mol at 25 °C
-
大气OH速率常数:7.70e-12 cm3/molecule*sec
-
自燃温度:748 °F (398 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:/p>PEG 400: 105 to 130 mPa.s at 20 °C; PEG 3000: 75 to 100 mPa.s at 20 °C; PEG 3350: 83 to 120 mPa.s at 20 °C; PEG 4000: 110 to 170 mPa.s at 20 °C; PEG 6000: 200 to 270 mPa.s at 20 °C; PEG 8000: 260 to 510 mPa.s at 20 °C; For polyethylene glycols having a average molecular weight greater than 400, the viscosity is determined on a 50 per cent m/m solution of the candidate substance in water
-
燃烧热:1189.2 kJ/mol
-
汽化热:50.5 kJ/mol
-
表面张力:47.99 mN/m at 25 °C; 45.76 mN/m at 50 °C; 43.54 mN/m at 75 °C; 41.31 mN/m at 100 °C
-
气味阈值:Odor index: 3 at 20 °C
-
折光率:Index of refraction: 1.4318 at 20 °C
-
解离常数:pKa = 15.1
-
相对蒸发率:<0.01; BuOAc = 1 at 90 % evaporation
-
保留指数:705 ;726 ;664.8 ;726 ;670 ;670 ;672
-
稳定性/保质期:
-
避免与强氧化剂、强酸接触。该物质是可燃性液体,对金属无腐蚀作用,但因其吸湿性强,应密封贮存。低温场所应采取保温措施,防止黏度上升和凝固。着火时可用泡沫灭火剂、二氧化碳或干粉灭火器扑救;误服者需立即用1∶2000高锰酸钾溶液洗胃并导泻,严重中毒者应送医院治疗。操作人员应佩戴防护装备,并定期进行体检,尤其是尿常规检查。
-
化学性质
- 与有机酸或无机酸反应生成酯类物质,如二元酸反应可生成聚酯纤维或树脂;与盐酸反应生成2-氯乙醇;硝酸作用下形成硝酸酯。
- 通过醚化反应,该物质与硫酸烷基酯及氢氧化钠反应生成相应的烷基醚。
- 在酸性催化剂下,缩醛的生成涉及醛类化合物的环合。
- 脱水过程中使用硫酸或氧化锌脱除水分,产生二元醇、乙醛及其他如巴豆醛等有机物。
- 氧化反应包括硝酸氧化及气相氧化,可形成乙二醛、羟基乙酸和草酸等多种化合物。
- 经碱金属或碱性氢氧化物处理后生成醇金属。
- 与环氧乙烷反应,最终产物为二甘醇、三甘醇甚至更高分子量的聚乙二醇。
-
对低级脊椎动物毒性较低,但在人类身上表现不同。由于沸点高及蒸气压低的特点,在一般情况下吸入中毒较少见,未破损皮肤渗透率也较低。但大量摄入(例如不足50克)可引发中枢神经系统刺激症状,包括呕吐、疲倦、昏睡、呼吸困难、震颤等,并可能造成肾脏充血和出血、脂肪肝、尿闭及支气管炎、肺炎等症状,最终导致死亡。大鼠、豚鼠和小鼠的经口半数致死剂量分别为8.54g/kg、6.61g/kg 和13.79mL/kg;人剂量约为1.4mL/kg 或100mL。误服者应立即用1∶2000高锰酸钾溶液洗胃和导泻,严重中毒者需送医治疗。
-
体内吸入乙二醇蒸气有害健康,对眼睛有刺激作用;长期暴露可能导致肾脏或神经系统损伤。操作时应在通风橱内进行以确保安全。
-
乙二醇具有毒性,能引起恶心、呕吐等胃肠道反应;较大剂量摄入可导致昏迷和抽搐症状。
-
计算性质
-
辛醇/水分配系数(LogP):-1.4
-
重原子数:4
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S26
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2905310000
-
危险品运输编号:UN 1219 3/PG 2
-
RTECS号:KW2975000
-
包装等级:Z01
-
危险标志:GHS07,GHS08
-
危险性描述:H302,H373
-
危险性防范说明:P260,P301 + P312 + P330
-
储存条件:1. 储存在阴凉、通风的仓库中,并远离火源和热源。与氧化剂、酸类分开存放,避免混合储存。配备相应的消防设备。储区应备有泄漏应急处理设备及合适的收容材料。由于吸湿性较强,需密封存储,长期保存时建议使用氮气密封。可用铁、软钢、铜或铝制容器储存,但长时间储存时宜选用涂覆的钢、铝或不锈钢容器。 2. 乙二醇容器上应标有“有毒”字样,以防误服和吸入其蒸气。操作人员需穿戴防护装备,并定期进行体检,特别是尿常规检查。 包装采用镀锌铁桶,每桶装100公斤或200公斤。存储时应确保密封,并在长期保存时进行氮封、防潮、防火和防冻处理。按易燃化学品规定进行储存与运输。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 乙二醇;甘醇 |
| 化学品英文名称: | Ethylene glycol |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 107-21-1 |
| 分子式: | C 2 H 602 |
| 分子量: | 62.07 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:乙二醇;甘醇 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 国内未见本品急慢性中毒报道。国外的急性中毒多系因误报。吸入中毒表现为反复发作性昏厥,并可有眼球震颤,淋巴细胞增多。口服后急性中毒分三个阶段;第一阶段主要为中枢神经系统症状,轻者似乙醇中毒表现,重者迅速产生昏迷抽搐,最后死亡;第二阶段,心肺症状明显,严重病例可有肺水肿,支气管肺炎,心力衰竭,第三阶段主要表现为不同程度肾功能衰竭。人的本品一次口服致死量估计为1.4ml/kg(1.56g/kg)。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用流动清水冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗15分钟。 |
| 吸入: | 迅速脱离现场至空气新鲜处。立即就医。 |
| 食入: | 误服者用大量水或饱和苏打水洗胃。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热或与氧化剂接触,有引起燃烧爆炸的危险。若遇高热,容器内压增大,有开裂和爆炸的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 110 |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | 3.2 |
| 爆炸上限[%(V/V)]: | 15.3 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自吸过滤式防毒面具(全面罩),穿一般作业工作服。尽可能切断泄漏源。防止流入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,提供良好的自然通风条件。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,戴防化学品手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、酸类接触。搬运时轻装轻卸,保持包装完整,防止洒漏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、酸类分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中国MAC(mg/m3): 20 苏联MAC:5mg/m3美国TLVWN: ACGIH 100mg/m3[上限值] |
| 监测方法: | 气相色谱法 |
| 工程控制: | 提供良好的自然通风条件。 |
| 呼吸系统防护: | 一般不需要特殊防护,高浓度接触时可佩带自给式呼吸器。 |
| 眼睛防护: | 必要时戴安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 必要时戴防化学品手套。 |
| 其他防护: | 工作后,淋浴更衣。避免长期反复接触。定期体检。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色、无臭、有甜味、粘稠液体。 |
| pH: | |
| 熔点(℃): | -13.2 |
| 沸点(℃): | 197.5 |
| 相对密度(水=1): | 1.11 |
| 相对蒸气密度(空气=1): | 2.14 |
| 饱和蒸气压(kPa): | 6.21(20℃) |
| 燃烧热(kJ/mol): | 281.9 |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 110 |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | 15.3 |
| 爆炸下限%(V/V): | 3.2 |
| 分子式: | C 2 H 602 |
| 分子量: | 62.07 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 与水混溶,可混溶于乙醇、醚等。 |
| 主要用途: | 用于制造树脂、增塑剂、合成纤维、化妆品和炸药,并用作溶剂、配制发动机的抗冻剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强酸。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 属低毒类 LD50:小鼠经口:8.0-15.3g/kg,大鼠经口:5.9-13.4g/kg LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。防止阳光直射。保持容器密封。应与氧化剂、酸类分开存放。搬运时轻装轻卸,保持包装完整,防止洒漏。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
乙二醇的生产方法主要包括以下几种:
这是目前工业规模生产乙二醇的主要方法。该过程是将环氧乙烷和水在加压(2.23MPa)和190-200℃条件下,在管式反应器中进行直接液相水合制得乙二醇,同时副产一缩二乙二醇、二缩三乙二醇和多缩聚乙二醇。所得乙二醇稀溶液经浓缩后精制得到合格的产品。
环氧乙烷与水在硫酸催化下,在60-80℃、9.806-19.61kPa的压力下进行水合反应,生成乙二醇。这种方法是早期开发的方法,但由于存在腐蚀、污染和产品质量问题,现在已被直接水合法取代。
这是一种不经环氧乙烷而直接从乙烯合成乙二醇的方法。该过程在催化剂(如氧化锑TeO2,钯催化剂)的存在下,在乙酸溶液中进行乙烯的氧化,生成单乙酸酯或二乙酸酯,进一步水解均得乙二醇。
此方法是通过二氯乙烷与水反应生成氯乙醇,然后通过水解得到乙二醇。这种方法较少使用。
- 甲醛法
上下游信息
反应信息
-
作为反应物:描述:乙二醇 以83.3的产率得到N-辛基单氧乙烯参考文献:名称:DP-b99的合成方法摘要:本发明涉及DP-b99的合成方法,包括将邻硝基苯酚A和碳酸钾加入溶剂中溶解,加入1,2-二溴乙烷得B;将B溶于溶剂中并加入高压釜在钯碳氢气环境下,加热反应得C;将C与二异丙基丙胺、碘化钠、溴乙酸乙脂在乙腈中混合,在氮气保护下,在80~90℃回流反应得D;将D加入乙醇中,滴加氢氧化钾的水溶液,室温下反应,pH2-4,析出E;将E加入到含吡啶的乙酸酐中,加热反应至完全,加乙醚,沉淀为F;室温下将钠加入乙二醇中,滴加1-溴辛烷,加热反应,加水,乙醚萃取得I;将F与I混合,加热反应加乙醚,静置,沉淀为DP-b99。优点是:还原硝基采取氢化法,反应清洁、便于操作、收率较高;用碘化钠和缚酸剂,使反应易进行,收率提高。公开号:CN102030670A
-
作为产物:参考文献:名称:[EN] PROCESS FOR REMOVING FORMALDEHYDE FROM A COMPOSITION COMPRISING GLYCOLALDHEDYDE
[FR] PROCÉDÉ POUR ÉLIMINER LE FORMALDÉHYDE D'UNE COMPOSITION COMPRENANT DU GLYCOLALDÉHYDE摘要:一种用于减少含有甘醛的组合物中甲醛重量百分比的方法,其中甲醛被转化为一个或多个甲醛缩醛,并在至少一种醇和催化剂的存在下通过反应精馏从反应精馏反应溶液中去除。公开号:WO2014131743A1 -
作为试剂:参考文献:名称:1, 1'-氧双(2, 6, 7-三氧杂-1-硅双环[2. 2. 2]辛-4-胺)及其合成方法摘要:本发明涉及1,1'‑氧双(2,6,7‑三氧杂‑1‑硅双环[2.2.2]辛‑4‑胺)及其合成方法。步骤如下:将硅源和三(羟甲基)氨基甲烷加入到溶剂中,氮气置换后反应至完全溶解。减压蒸馏后冷却,加入乙醇,升温搅拌,过滤。滤液加至有机溶剂中析出固体,搅拌,过滤,洗涤,烘干,得到新型含氮有机硅化合物。公开号:CN118221713A
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
Solid-phase synthesis of C-terminal peptide aldehydes from amino acetals anchored to a backbone amide linker (BAL) handle
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
[EN] CATHEPSIN CYSTEINE PROTEASE INHIBITORS<br/>[FR] INHIBITEURS DE PROTÉASES À CYSTÉINE DE TYPE CATHEPSINES申请人:MERCK SHARP & DOHME公开号:WO2015054038A1公开(公告)日:2015-04-16This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
表征谱图
-
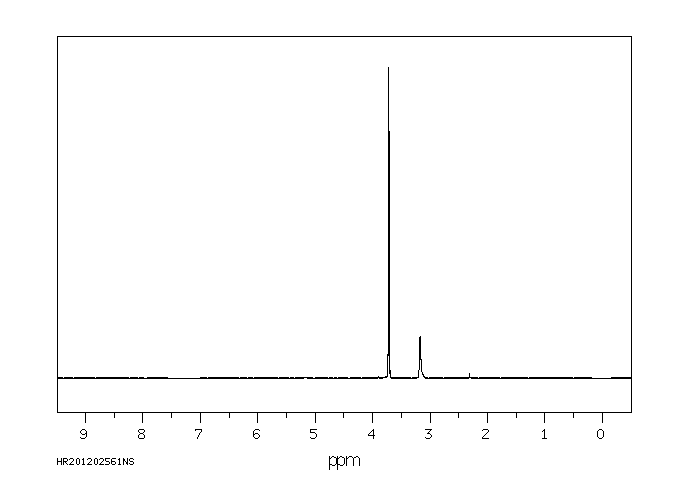
氢谱1HNMR
-
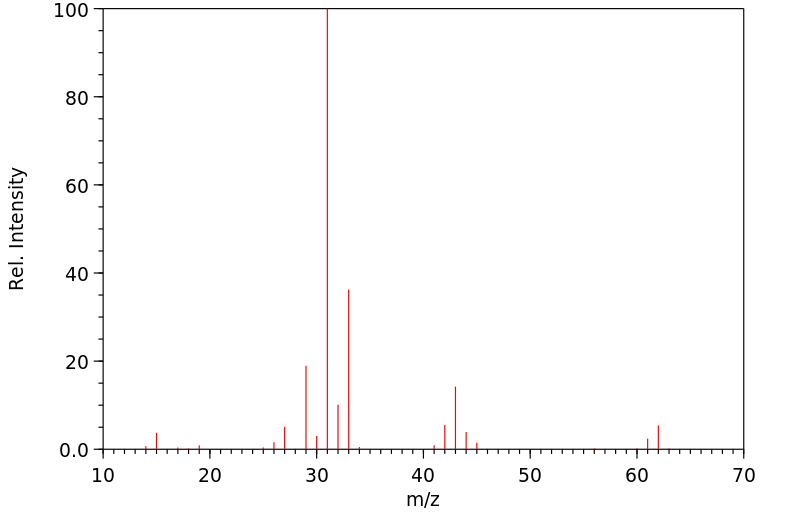
质谱MS
-
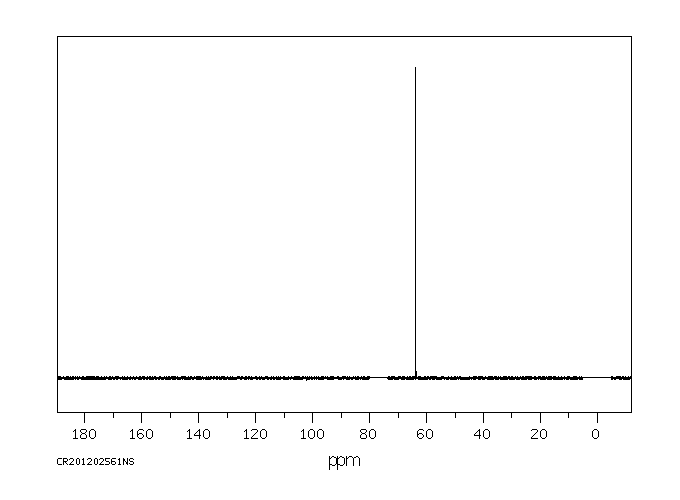
碳谱13CNMR
-
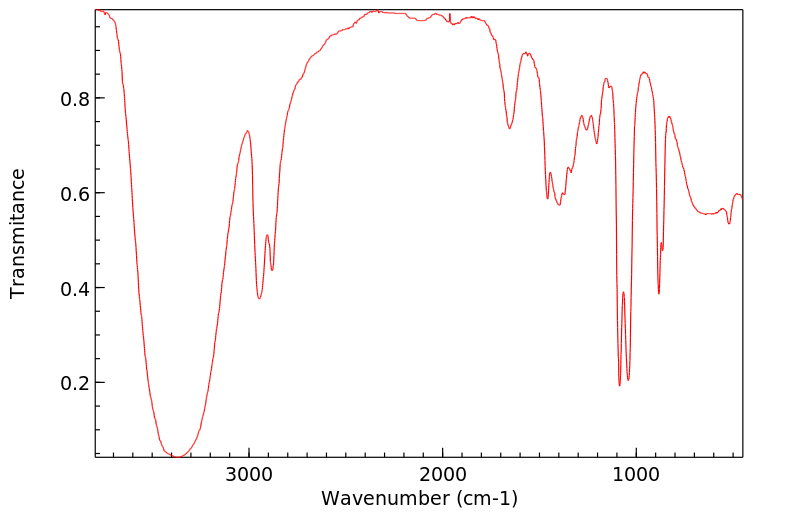
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息