二苯甲酮肟 | 574-66-3
中文名称
二苯甲酮肟
中文别名
二苯酮肟;苯甲酮肟
英文名称
Benzophenone oxime
英文别名
diphenylmethanone oxime;N-benzhydrylidenehydroxylamine
CAS
574-66-3
化学式
C13H11NO
mdl
MFCD00051461
分子量
197.236
InChiKey
DNYZBFWKVMKMRM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-144 °C
-
沸点:334.34°C (rough estimate)
-
密度:1.0957 (rough estimate)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2928000090
-
危险品运输编号:HAZARD
-
RTECS号:DJ1810000
-
包装等级:III
-
危险类别:4.1,6.1
-
危险性防范说明:P240,P210,P241,P280,P370+P378,P501,P261,P270,P271,P264,P337+P313,P305+P351+P338,P361+P364,P332+P313,P301+P310+P330,P302+P352+P312,P304+P340+P311,P403+P233,P405
-
危险性描述:H228,H301+H311+H331,H315,H319
-
储存条件:贮存: 将密器密封后,放入密封的主容器中,并存放在阴凉、干燥的地方。
SDS
| Name: | Benzophenone Oxime 98% Material Safety Data Sheet |
| Synonym: | Diphenylketone Oxime |
| CAS: | 574-66-3 |
Synonym:Diphenylketone Oxime
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 574-66-3 | Benzophenone Oxime | 98% | 209-373-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 574-66-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 141-143 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density:
Molecular Formula: C6H5CNOH(C6H5)
Molecular Weight: 197.0831
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 574-66-3: DJ1810000 LD50/LC50:
Not available.
Carcinogenicity:
Benzophenone Oxime - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 574-66-3: No information available.
Canada
CAS# 574-66-3 is listed on Canada's NDSL List.
CAS# 574-66-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 574-66-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二苯甲酮亚胺 Benzophenone imine 1013-88-3 C13H11N 181.237 —— Benzophenon-(O-benzyl-oxim) 3362-43-4 C20H17NO 287.361 —— c-phenyl-N-(t-butyl)-c-phenylnitrone 58796-23-9 C17H19NO 253.344 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— benzophenone oxime methyl ether 3376-34-9 C14H13NO 211.263 —— N-(benzhydrylideneamino)oxy-1,1-diphenylmethanimine 7463-88-9 C26H20N2O 376.458 二苯甲酮-(O-乙基肟) benzophenon-(O-ethyl oxime ) 58133-21-4 C15H15NO 225.29 二苯甲酮亚胺 Benzophenone imine 1013-88-3 C13H11N 181.237 —— Benzophenonoxim-O-methylthiomethylaether 25056-49-9 C15H15NOS 257.356 —— benzophenone O-allyloxime 163224-06-4 C16H15NO 237.301 —— Benzophenonoxim-O-isopropylether 61582-66-9 C16H17NO 239.317 —— benzophenone-O-prop-2-ynyl oxime —— C16H13NO 235.285 —— 1,1-diphenyl-N-[(E)-prop-1-enoxy]methanimine 1453804-71-1 C16H15NO 237.301 —— diphenylmethanone, O-(2-chloroethyl)oxime —— C15H14ClNO 259.735 —— O-(2-aminoethyl)benzophenone oxime —— C15H16N2O 240.305 N-(二苯基亚甲基)甲胺N-氧化物 N-methyl-diphenyl-nitrone 7500-79-0 C14H13NO 211.263 —— Diphenyl-methanone O-(3-hydroxy-propyl)-oxime 916666-99-4 C16H17NO2 255.316 —— diphenylmethanone O-(2-(methylamino)ethyl)oxime 953772-61-7 C16H18N2O 254.332 —— benzophenone oxime O-trimethylsilyl ether 17922-31-5 C16H19NOSi 269.418 —— O-(3-aminopropyl)benzophenone oxime —— C16H18N2O 254.332 —— benzophenone O-acetyl oxime 21160-02-1 C15H13NO2 239.274 —— benzophenone O-(chloroformyl)oxime 18304-44-4 C14H10ClNO2 259.692 —— O-(2-dimethylaminoethyl)benzophenone oxime 3362-42-3 C17H20N2O 268.359 —— benzophenone O-(2-hydroxypropyl)oxime 94460-69-2 C16H17NO2 255.316 —— 2-(diphenylmethylenaminooxy)acetic acid 56483-66-0 C15H13NO3 255.273 —— O-4-pentenyl benzophenone oxime —— C18H19NO 265.355 —— N-[(E)-3-methylbut-1-enoxy]-1,1-diphenylmethanimine 1453804-74-4 C18H19NO 265.355 —— benzophenone O-(3-N-methylaminopropyl)oxime 953772-63-9 C17H20N2O 268.359 —— N-[(E)-hex-1-enoxy]-1,1-diphenylmethanimine 1453804-72-2 C19H21NO 279.382 —— β-(N-Diphenylmethylen-aminooxy)-propionsaeure 15985-45-2 C16H15NO3 269.3 —— Benzophenon-(O-benzyl-oxim) 3362-43-4 C20H17NO 287.361 —— O-(3-dimethylaminopropyl)benzophenone oxime 846-93-5 C18H22N2O 282.385 —— {[(Diphenylmethylidene)amino]oxy}(methylamino)methanone 22001-32-7 C15H14N2O2 254.288 —— diphenylmethanone O-propionyl oxime —— C16H15NO2 253.301 二苯甲酮腙 benzophenone hydrazone 5350-57-2 C13H12N2 196.252 —— N-[(E)-oct-1-enoxy]-1,1-diphenylmethanimine 1453804-73-3 C21H25NO 307.436 —— O-(1,2-Dimethoxypropyl)benzophenone oxime —— C18H21NO 267.371 —— (3-amino-2-hydroxypropyl)oximino-diphenylmethylene —— C16H18N2O2 270.331 —— γ-(N-Diphenylmethylen-aminooxy)-buttersaeure 15985-47-4 C17H17NO3 283.327 —— benzophenone O-phenethyloxime 1233086-73-1 C21H19NO 301.388 —— O-(4-dimethylaminobutyl)benzophenone oxime 285551-58-8 C19H24N2O 296.412 —— O-phenylbenzophenone oxime 29127-87-5 C19H15NO 273.334 —— 6-[[(diphenylmethylene)amino]oxy]hexanoic acid 15985-49-6 C19H21NO3 311.381 —— N-{[(Diphenylmethylidene)amino]oxy}(diphenyl)methanimine N-oxide 7463-89-0 C26H20N2O2 392.457 —— benzophenone-0-(2,3-epoxypropyl)-oxime 60979-65-9 C16H15NO2 253.301 —— 2-(Benzhydrylideneamino)oxyacetohydrazide 56562-34-6 C15H15N3O2 269.303 —— Methyl 3-(benzhydrylideneamino)oxypropanoate 211572-91-7 C17H17NO3 283.327 —— benzophenone oxime butanoate —— C17H17NO2 267.327 —— 3-(Benzhydrylideneamino)oxypropanehydrazide 201597-38-8 C16H17N3O2 283.33 —— 2-(diphenylmethylenaminooxy)acetic acid ethyl ester 149691-57-6 C17H17NO3 283.327 —— (3-methylamino-2-hydroxypropyl)oximino-diphenylmethylene —— C17H20N2O2 284.358 —— diphenylmethanone O-pivaloyl oxime 1234464-18-6 C18H19NO2 281.354 —— benzophenone O-(ethyl E-2-propenoate)oxime —— C18H17NO3 295.338 —— benzophenone O-(2-hydroxyoctyl)oxime 1068000-65-6 C21H27NO2 325.451 —— benzophenone O-(2-morpholin-4-yl-ethyl)-oxime 98883-94-4 C19H22N2O2 310.396 甲胺,N-(二苯亚甲基)-1-(甲硫基)-,N-氧化 benzhydrylidene-methylsulfanylmethyl-amine oxide 19133-01-8 C15H15NOS 257.356 —— Ethyl 6-(benzhydrylideneamino)oxyhexanoate 436157-25-4 C21H25NO3 339.434 —— N-(diphenylmethylene)cyanamide 34414-10-3 C14H10N2 206.247 —— benzophenone O-(3-butoxy-2-hydroxypropyl)oxime 1068000-49-6 C20H25NO3 327.423 —— diphenylmethanone O-(3,3-dimethylbutanoyl) oxime —— C19H21NO2 295.381 —— benzophenone O-(3-allyloxy-2-hydroxypropyl)oxime 1068000-35-0 C19H21NO3 311.381 —— O-nonanoyl benzophenone oxime —— C22H27NO2 337.462 —— bis(diphenylmethyleneamino) monosulphide 15972-30-2 C26H20N2S 392.524 —— 1-(Diphenylmethylen)iminoxy-3-isopropylamino-2-propanol 56442-83-2 C19H24N2O2 312.412 二苯甲酮吖嗪 benzophenone azine 983-79-9 C26H20N2 360.458 —— benzophenone azine monoxide 7463-87-8 C26H20N2O 376.458 —— O-Benzhydryl-benzophenon-oxim 65311-52-6 C26H21NO 363.459 —— benzophenone O-(1H-imidazol-1-yl)methyloxime 1357296-05-9 C17H15N3O 277.326 二苯亚甲基氨基乙腈 N-(diphenylmethylene)aminoacetonitrile 70591-20-7 C15H12N2 220.274 —— benzophenone O-[(R)-3-pyrrolidinyl]oxime 952747-31-8 C17H18N2O 266.343 —— N-tert-butyl-1,1-diphenylmethanimine 27126-13-2 C17H19N 237.345 邻苯乙酰基二苯甲酮肟 O-phenylacetyl benzophenone oxime 19347-13-8 C21H17NO2 315.371 3-[(二苯基亚甲基)氨基]丙腈 3-((diphenylmethylene)amino)propanenitrile 74687-07-3 C16H14N2 234.301 二苯甲酮肟苯甲酰酯 benzophenone O-benzoyloxime 3362-33-2 C20H15NO2 301.345 —— benzophenone O-(2-hydroxy-2-phenylethyl)oxime 94869-93-9 C21H19NO2 317.387 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
反应信息
-
作为反应物:参考文献:名称:肟酯在苯和吡啶中的光化学芳基化:联芳基化合物的简单合成摘要:在苯和吡啶中照射二苯甲酮O-芳烃羰基肟,分别以高收率得到相应的芳基苯和芳基吡啶。DOI:10.1016/s0040-4039(01)91195-5
-
作为产物:描述:参考文献:名称:Base and solvent mediated decomposition of tosylhydrazones: highly selective synthesis of N-alkyl substituted hydrazones, dialkylidenehydrazines, and oximes摘要:Base and solvent mediated decomposition of tosylhydrazones was studied. It was found that reaction of tosylhydrazones in CH3NO2 in the presence of 1 equiv of K2CO3 in 90 degrees C gave N-alkylated products in 52-96% yield. However, when the same reaction was carried out in mixed solvent of CH3NO2 and dioxane in the presence of 3 equiv of NaOH at 110 degrees C, dialkylidenehydrazines were obtained in moderate to high yield. If the reaction was carried out in mixed solvent of CH3NO2 and DMSO in the presence of 10 equiv of NaOH at 110 degrees C, CH3NO2 can act as the precursor of hydroxylamine and corresponding oximes were formed in up to 92% yield. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2013.03.055
-
作为试剂:描述:参考文献:名称:Photochemistry of diaryl ketones: a new photocyclization reaction摘要:DOI:10.1021/jo00161a030
文献信息
-
Aren- und cyclopentadienyl-halbsandwichkomplexe des rutheniums mit oximaten, carboxylaten, iminen und azavinylidenen als liganden作者:H. Werner、T. Daniel、W. Knaup、O. NürnbergDOI:10.1016/0022-328x(93)83372-3日期:1993.12The reaction of [C6H6Ru(PiPr3)Cl2] (1) with NaONCRR′ in the presence of KPF6 leads to the formation of the oximatoruthenium(II) complexes [C6H6Ru(η2-ONCRR′)(PiPr3)]PF6 (2–5) in 70–90% yield. Compound 5 (R Me, R′ tBu) reacts with HNCPh2 via ligand exchange to give [C6H6Ru(NCPh2)(Pi Pr3)]PF6 (8). The azavinylidene complex 8 has also been prepared from the acetatoruthenium derivative [C6H6Ru(η2-O2在KPF 6存在下,[C 6 H 6 Ru(P i Pr 3)Cl 2 ](1)与NaONCRR'的反应导致形成氧亚氨基钌(II)络合物[C 6 H 6 Ru( η 2 -ONCRR')(P我镨3)] PF 6(2 - 5)在70-90%的产率。化合物5(RMe中,R'吨卜)与HNCPh发生反应2通过配体交换,得到[C 6 H ^ 6的Ru(NCPh 2)(Pi Pr 3)] PF 6(8)。所述azavinylidene复杂8也被从acetatoruthenium衍生物制备[C 6 H ^ 6的Ru(η 2 -O 2 CCH 3)(P我镨3)] PF 6(6),其可以或者从可以得到1,CH 3 CO 2 Na和KPF 6或从治疗的5用过量的CH 3 CO 2 H的hexamethylbenzeneruthenium化合物的合成[C6我6的Ru(NCR' 2)PR 3 ])PF
-
Iron-Catalyzed Synthesis of 2<i>H</i>-Imidazoles from Oxime Acetates and Vinyl Azides under Redox-Neutral Conditions作者:Zhongzhi Zhu、Xiaodong Tang、Jianxiao Li、Xianwei Li、Wanqing Wu、Guohua Deng、Huanfeng JiangDOI:10.1021/acs.orglett.7b00203日期:2017.3.17A novel and versatile method for the synthesis of 2H-imidazoles via iron-catalyzed [3 + 2] annulation from readily available oxime acetates with vinyl azides has been developed. This denitrogenative process involved N–O/N–N bond cleavages and two C–N bond formations to furnish 2,4-substituted 2H-imidazoles. This protocol was performed under mild reaction conditions and needed no additives or ligands
-
Deoximation of Oxime<i>O</i>-Acetates, Oximes, and Oxime Ethers by Nonacarbonyldiiron or Pentacarbonyliron. An Electronic Effect for the N–O Bond Cleavage作者:Makoto Nitta、Ichiro Sasaki、Hiroyuki Miyano、Tomoshige KobayashiDOI:10.1246/bcsj.57.3357日期:1984.11The reaction of [Fe2(CO)9] or [Fe(CO)5] with oxime O-acetates, oximes, and oxime ethers under photoirradiation or thermal conditions undergoes deoximation to give the corresponding ketones. The oxime O-acetate was found to be the most reactive class of these compounds. A proposed mechanism involves an initial complexation of the [Fe(CO)4] species to the nitrogen atom of the oxime group, and the subsequent
-
SO<sub>2</sub> F<sub>2</sub> -Activated Efficient Beckmann Rearrangement of Ketoximes for Accessing Amides and Lactams作者:Guofu Zhang、Yiyong Zhao、Lidi Xuan、Chengrong DingDOI:10.1002/ejoc.201900844日期:2019.8.15A novel protocol for the efficient activation of the Beckmann rearrangement utilizing the readily available sulfuryl fluoride (SO2F2 gas) is reported. The substrate scope of this methodology has been demonstrated by 37 examples with good to nearly quantitative isolated yields in a short time. A tentative mechanism was proposed involving formation and elimination of sulfonyl ester.报道了一种利用容易获得的硫酰氟(SO 2 F 2气体)有效激活贝克曼重排的新方案。该方法的底物范围已通过37个实例证明,并在短时间内获得了良好至近乎定量的分离产率。提出了一种尝试性的机制,涉及形成和消除磺酰基酯。
-
On the mixed oxides-supported niobium catalyst towards benzylamine oxidation作者:Álisson Silva Granato、Gustavo S. Gonçalves de Carvalho、Carla G. Fonseca、Javier Adrio、Alexandre A. Leitão、Giovanni Wilson AmaranteDOI:10.1016/j.cattod.2020.08.011日期:2021.12synthesized and applied towards oxidation reactions of benzylamine derivatives. Under the optimized reaction conditions, the selectivity to oxime enhanced, leading to the main product with up to 72 %. Moreover, even α-substituted benzylamines were well tolerated and led to oximes in good isolated yields. It is important to mention; four equivalents of the harmless and inexpensive hydrogen peroxide were employed
表征谱图
-
氢谱1HNMR
-
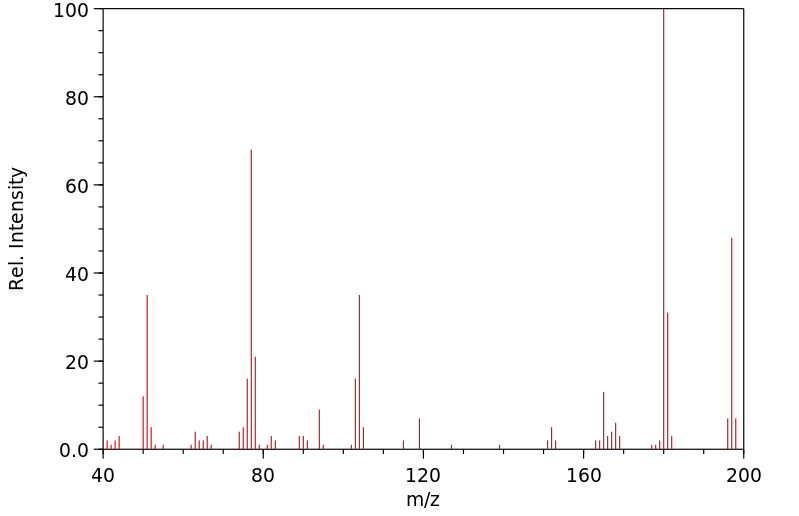
质谱MS
-
碳谱13CNMR
-
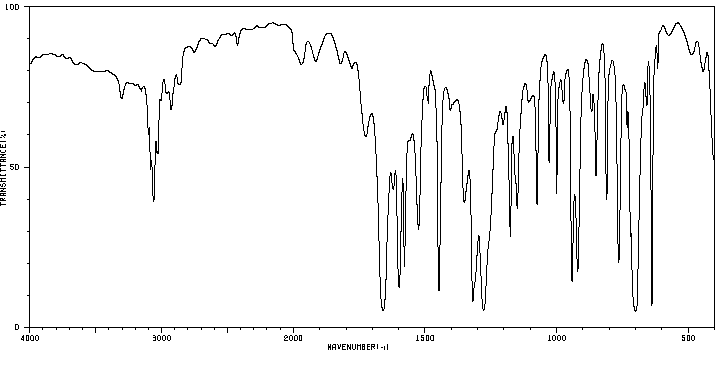
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫