全氟辛酸 | 335-67-1
中文名称
全氟辛酸
中文别名
十五氟八碳酸;十五氟辛酸水合物;全氟正辛酸;十五氟辛酸
英文名称
Perfluorooctanoic acid
英文别名
PFOA;pentadecafluorooctanoic acid;perfluoro octanoate;2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctanoic acid
CAS
335-67-1
化学式
C8HF15O2
mdl
MFCD00004174
分子量
414.071
InChiKey
SNGREZUHAYWORS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55-56 °C (lit.)
-
沸点:189 °C/736 mmHg (lit.)
-
密度:1,7 g/cm3
-
闪点:189-192°C
-
溶解度:3.4g/l
-
物理描述:Solid
-
颜色/状态:White to off-white powder
-
蒸汽压力:3.16X10-2 mm Hg at 25 °C
-
稳定性/保质期:
本品为强酸性,呈现白色鳞状结晶。其蒸气刺激皮肤、眼睛及黏膜,在加热至250℃时可释放毒性气体。因此,生产设备应确保密闭,并在车间安装良好的通风设施。操作人员需穿戴防护用具以保障安全。
-
分解:When heated to decomposition it emits toxic vapors of /flourine/.
-
表面张力:15.2 dynes/cm
-
解离常数:pKa = 1.30
-
保留指数:1045
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:25
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:17
ADMET
代谢
无靶向代谢组学对实验动物和接触化学物质的人体体液的检测,可以揭示毒性早期的迹象并指出毒性途径。鸟胚胎与母体分离发育,这为研究化学物质在胚胎发育过程中的影响提供了独特的可能性,同时最小化了来自母体的混淆因素。在本研究中,我们探索了鸡胚胎的血液血浆和尿囊液作为揭示胚胎发育过程中化学物质暴露引起的代谢变化的基质。胚胎在第7天通过卵注射暴露于环境污染物全氟辛酸(PFOA),并在第12天比较了其对代谢轮廓的影响与GW7647和罗格列酮引起的影响,这两种物质分别是过氧化物酶体增殖激活受体alpha(PPARalpha)和PPARgamma的选择性激动剂。通过正交偏最小二乘判别分析(OPLS-DA)对尿囊液中的代谢物浓度进行分析,结果显示,暴露于GW7647、罗格列酮和车辆对照的胚胎之间有明显的分离。在血液血浆中,只有GW7647对代谢轮廓产生了显著影响。PFOA在最高剂量下引起了胚胎死亡并增加了相对肝重。PFOA的亚致死剂量在任一基质中并未显著影响代谢轮廓,尽管某些单一代谢物似乎有所改变。PFOA在小鼠新生儿中的死亡已被建议通过激活PPARalpha介导。然而,我们发现鸡胚胎暴露于PFOA的代谢物轮廓与暴露于PPAR激动剂的胚胎并不相似。这表明PFOA在我们的模型中,在远高于野生鸟类发现的卵和胚胎浓度下,并未激活PPAR途径。目前的研究表明,鸡胚胎的尿囊液和血浆是有用的且互补的基质,用于探索化学物质在胚胎发育过程中暴露对代谢轮廓的影响。
Untargeted metabolic profiling of body fluids in experimental animals and humans exposed to chemicals may reveal early signs of toxicity and indicate toxicity pathways. Avian embryos develop separately from their mothers, which gives unique possibilities to study effects of chemicals during embryo development with minimal confounding factors from the mother. In this study we explored blood plasma and allantoic fluid from chicken embryos as matrices for revealing metabolic changes caused by exposure to chemicals during embryonic development. Embryos were exposed via egg injection on day 7 to the environmental pollutant perfluorooctanoic acid (PFOA), and effects on the metabolic profile on day 12 were compared with those caused by GW7647 and rosiglitazone, which are selective agonists to peroxisome-proliferator activated receptor alpha (PPARalpha) and PPARgamma, respectively. Analysis of the metabolite concentrations from allantoic fluid by Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) showed clear separation between the embryos exposed to GW7647, rosiglitazone, and vehicle control, respectively. In blood plasma only GW7647 caused a significant effect on the metabolic profile. PFOA induced embryo mortality and increased relative liver weight at the highest dose. Sublethal doses of PFOA did not significantly affect the metabolic profile in either matrix, although single metabolites appeared to be altered. Neonatal mortality by PFOA in the mouse has been suggested to be mediated via activation of PPARalpha. However, we found no similarity in the metabolite profile of chicken embryos exposed to PFOA with those of embryos exposed to PPAR agonists. This indicates that PFOA does not activate PPAR pathways in our model at concentrations in eggs and embryos well above those found in wild birds. The present study suggests that allantoic fluid and plasma from chicken embryos are useful and complementary matrices for exploring effects on the metabolic profile resulting from chemical exposure during embryonic development.
来源:Hazardous Substances Data Bank (HSDB)
代谢
全氟辛酸(PFOA)不被代谢,且有证据表明该化合物在肠肝之间循环。
PFOA is not metabolized and there is evidence of enterohepatic circulation of the compound.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
全氟辛酸(PFOA)是一种白色至略带白色的粉末。PFOA主要用于生产其盐类,这些盐类在氟聚合物和氟弹性体的生产中作为必不可少的加工助剂。PFOA还用作防水剂、消防泡沫中的表面活性剂,以及合成氟丙烯酸酯的中间体。它在制造特氟龙和类似化学品(称为氟碳聚合物)的过程中使用,尽管在生产过程中会被烧掉,在最终产品中,包括特氟龙涂层产品中,不会以显著量存在。人类暴露和毒性:一项甲状腺研究结果表明,全氟烷基物质与老年人群中甲状腺激素水平的微妙变化有关,这些关联可能因年龄而异。暴露于PFOA可能会降低生育能力。在明尼苏达州Cottage Grove的一家工厂,那些在可能暴露于PFOA的工作中工作了27年的工人,或者那些有明确PFOA暴露9年的人,死于脑血管疾病的可能性比普通人群高出3.3倍。根据对两个不同3M工厂的工人进行研究,PFOA与血清胆固醇和甘油三酯水平随时间有显著的正相关。有人建议,PFOA水平与ADHD之间存在显著的剂量反应关系。孕早期母体PFOA水平与胎儿腹围和出生长度较小有关。另一项研究中,母体PFOA浓度与儿童普通感冒的发作次数以及PFOA与胃肠炎发作次数之间存在正相关。在一项对男性和女性血清水平的研究中,与同一人群中的女性相比,男性的PFOA浓度更高。在150 uM PFOA暴露的HepG2细胞中,通过同时流式细胞术分析凋亡相关的DNA链断裂,揭示了DNA断裂,这是在核碎片化之前的。流行病学证据不支持PFOA暴露与人类癌症之间有因果关系的假设。然而,根据有限的癌症死亡人数,与不从事PFOA生产相比,从事PFOA暴露工作十年与前列腺癌死亡率增加3.3倍有关。动物研究:动物研究指出,PFOA可引起多种毒性效应,包括肝和脑功能障碍、致癌性以及生殖和发育毒性。在小鼠的皮肤暴露后,PFOA被证明具有免疫毒性。根据另一项针对小鼠的研究,PFOA可以诱导气道炎症并改变气道功能。对小鼠的组织学检查显示,连续14天暴露于PFOA导致严重的肝细胞损伤和明显的炎症细胞浸润。此外,PFOA处理在小鼠肝脏中显著诱导了丙二醛的形成和过氧化氢的生成,这些都是氧化应激的指标。此外,由PFOA暴露引起的肝脏中白细胞介素-6、环氧合酶-2和C反应蛋白的水平,这些炎症反应的标志物显著增加。对小鼠进行围产期暴露于PFOA的研究在成年后代中产生了代谢效应。PFOA诱导的男性生殖障碍可能涉及发育损伤和对小鼠睾丸中NRF2介导的抗氧化反应的抑制。在小鼠和大鼠中,PFOA单独并未引起任何神经毒性症状。然而,在小鼠新生儿暴露后描述了PFOA的发育神经毒性。新生儿暴露于PFOA影响了胆碱能系统,表现为对尼古丁的反应降低,而对照组对尼古丁的反应过度活跃。在Ames试验中,使用四种沙门氏菌typhimurium菌株,在存在或不存在代谢系统的情况下,PFOA并未表现出致突变性。小鼠对PFOA的短期高剂量暴露增强了对脂多糖(LPS)的炎症反应,LPS是一种强大的先天免疫激活剂。生态毒性研究:在罕见的小鱼中发现,PFOA改变了与脂质代谢和运输、激素作用、免疫反应、线粒体功能、脂肪酸生物合成和运输有关的基因。此外,PFOA抑制了负责甲状腺激素生物合成的基因,并显著诱导了雌激素响应基因。对果蝇的研究表明,PFOA在有机体水平上的毒性效应与有机体的发育状况有关,幼虫对这种化学物质最为敏感。
IDENTIFICATION AND USE: Perfluorooctanoic acid (PFOA) is a white to off-white powder. PFOA is used primarily to produce its salts, which are used as essential processing aids in the production of fluoropolymers and fluoroelastomers. PFOA is also used as a water repellent, a surfactant in firefighting foams, and as an intermediate in the synthesis of fluoroacrylic esters. It is used in the process of making Teflon and similar chemicals (known as fluorotelomers), although it is burned off during the process and is not present in significant amounts in the final products, including Teflon-coated products. HUMAN EXPOSURE AND TOXICITY: Results of a thyroid study suggest that perfluoroalkyl substances are associated with subtle alterations in thyroid hormone levels in aging populations, and that these associations are likely to vary by age. Exposure to PFOA may reduce fecundability. At a plant in Cottage Grove, Minnesota, workers with 27 years of exposure in probable PFOA exposed jobs or those with 9 years of definite PFOA exposure were 3.3 times more likely to die of cerebrovascular disease than the general population. Based on workers studied at two different 3M plants, there was a statistically significant positive association between PFOA and serum cholesterol and triglycerides levels over time. It has been suggested that there is a significant dose response relationship between PFOA levels and ADHD. Maternal PFOA levels in early pregnancy were associated with smaller abdominal circumference and birth length. In another study, there was a positive association between the maternal concentrations of PFOA and the number of episodes of common cold for the children, and between PFOA and the number of episodes of gastroenteritis. In a study on blood serum levels of men and women, higher concentrations of PFOA were observed in men in comparison to women from the same populations. Simultaneous flow cytometric analysis of apoptosis-associated DNA strand breaks revealed DNA breaks in HepG2 cells exposed to 150 uM PFOA, prior to nuclear fragmentation. Epidemiologic evidence does not support the hypothesis of a causal association between PFOA exposure and cancer in humans. However, ten years of employment in PFOA exposed jobs was associated with a 3.3-fold increase in prostate cancer mortality compared to no employment in PFOA production, based on limited number of cancer deaths. ANIMAL STUDIES: Animal studies have indicated that PFOA cause a wide array of toxic effects including liver and brain dysfunction, carcinogenicity and reproductive and developmental toxicity. PFOA was demonstrated to be immunotoxic following dermal exposure of mice. Based on another study in mice, PFOA can induce airway inflammation and alter airway function. Histological examination of mice showed that the exposure to PFOA for 14 consecutive days led to serious hepatocellular injury and obvious inflammatory cell infiltration. In addition, malondialdehyde formation and hydrogen peroxide generation, indicators of oxidative stress, were significantly induced by PFOA treatment in the liver of mice. Furthermore, hepatic levels of interleukin-6, cyclooxygenase-2, and C-reactive protein, markers of inflammatory response, were markedly increased by exposure to PFOA. A study with perinatal exposure to PFOA in mice produced metabolic effects in adult offspring. PFOA-induced male reproductive disorders might be involved in developmental impairment and inhibition of NRF2-mediated antioxidant response in the testis of mice. In mice and rats, PFOA alone did not cause any neurotoxic symptoms. However, developmental neurotoxicity of PFOA described after neonatal exposure in mice. Neonatal exposure to PFOA affected the cholinergic system, manifested as a hypoactive response to nicotine, compared to a hyperactive response to nicotine in controls. PFOA was not mutagenic in the Ames test, using four strains of Salmonella typhimurium in the presence or absence of metabolic system. High-dose, short-term exposure of mice to PFOA augments inflammatory responses to lipopolysaccharide (LPS), a potent activator of innate immunity. ECOTOXICITY STUDIES: In rare minnows, PFOA was found to alter genes involved in lipid metabolism and transport, hormone action, immune responses, mitochondrial functions, fatty acid biosynthesis, and transport. In addition, PFOA inhibited genes responsible for thyroid hormone biosynthesis and significantly induced estrogen-responsive genes. A study on fruit flies indicated that the toxic effects of PFOA at the organismal level were associated with the developmental status of the organism, with larvae being most sensitive to this chemical.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:全氟辛酸
IARC Carcinogenic Agent:Perfluorooctanoic acid
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:2B组:可能对人类致癌
IARC Carcinogenic Classes:Group 2B: Possibly carcinogenic to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第110卷:(2017年)某些用作溶剂和聚合物制造的化学品
IARC Monographs:Volume 110: (2017) Some Chemicals Used as Solvents and in Polymer Manufacture
来源:International Agency for Research on Cancer (IARC)
毒理性
2B,可能对人类有致癌性。
2B, possibly carcinogenic to humans. (L135)
来源:Toxin and Toxin Target Database (T3DB)
吸收、分配和排泄
被限制在呼吸计-代谢室中的虹鳟鱼(Oncorhynchus mykiss)通过动脉内(i.a.)注射接受了全氟辛酸(PFOA)的剂量,并取样以获得血浆、尿液和呼出水的浓度-时间过程数据。然后通过室模型分析这些数据以估计肾和鳃的清除率。对所有动物平均来看,肾清除率(1.35 mL/hr/kg)比鳃清除率(0.12 mL/hr/kg)高出十倍以上。平均整体消除半衰期为12.6天,这比之前使用较小虹鳟鱼的研究得到的值要长一些。通过在室暴露结束时收集组织以及在一个单独的组织时间过程实验中评估PFOA的组织分布。从时间过程研究中可以看出,内部稳态在i.a.注射后24小时内建立。与之前的研究一致,稳态时组织中PFOA浓度的等级顺序为:血浆>肝脏>肾脏>肌肉。在第二组室实验中,鱼在水中暴露于PFOA以确定鳃的摄取速率。鳃摄取速率太低,无法通过测量吸入和呼出水中的PFOA浓度直接评估。因此,通过室模型使用血浆浓度时间过程数据和从消除实验中得到的模型参数估计了摄取率常数(平均0.19 L/d/kg;0.1%的摄取效率)。从这个努力中可以清楚地看出,虹鳟鱼主要通过肾路线消除PFOA。这一发现与对哺乳动物的大量研究一致,并表明虹鳟鱼拥有促进PFOA从血浆到尿液移动的膜转运体。
Rainbow trout (Oncorhynchus mykiss) confined to respirometer-metabolism chambers were dosed with perfluorooctanoate (PFOA) by intra-arterial (i.a.) injection and sampled to obtain concentration time-course data for plasma, urine, and expired water. The data were then analyzed by compartmental modeling to estimate rates of renal and branchial clearance. Averaged across all animals, the renal clearance rate (1.35 mL/hr/kg) was more than ten times greater than the branchial clearance rate (0.12 mL/hr/kg). The average whole-body elimination half-life was 12.6 d, which is somewhat longer than values obtained in previous studies with smaller trout. The tissue distribution of PFOA was assessed by collecting tissues at the end of chambered exposures and in a separate tissue time-course experiment. From the time-course study it appeared that an internal steady-state was established within 24 hr of i.a. injection. Consistent with previous studies, the rank order of PFOA concentration in tissues at steady state was: plasma>liver>kidney>muscle. In a second set of chambered experiments, fish were exposed to PFOA in water to determine the rate of branchial uptake. Branchial uptake rates were too low to assess directly by measuring PFOA concentrations in inspired and expired water. Uptake rate constants (mean 0.19 L/d/kg; 0.1% uptake efficiency) were therefore estimated by compartmental modeling using plasma concentration time-course data and model parameters derived from the elimination experiments. It is clear from this effort that elimination of PFOA by trout occurs primarily via the renal route. This finding is consistent with numerous studies of mammals and suggests that trout possess membrane transporters that facilitate the movement of PFOA from plasma to urine.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
确定人体血液中毒物清除率的化学方法是毒代动力学暴露评估的一个关键组成部分。在考虑持续暴露的情况下,分析时间生物监测数据会导致计算出的表观消除半衰期值比固有值要长。固有消除半衰期仅仅是消除速率的函数,而表观消除半衰期反映了消除和持续暴露的过程。混淆固有半衰期和表观半衰期值可能会导致对生物监测数据的误解,并可能导致后续建模努力中的预测夸大。这项工作回顾了为计算固有和表观半衰期值而开发的一阶方程,以及由于混淆这两个值而可能产生的潜在偏差。使用这些方程分析已发表的关于全氟辛酸(PFOA)的人类生物监测数据,以提供低、中、高偏差的例子,用于确定从血浆或血清中固有消除半衰期,通常分析血液的PFOA组分。还提供了一种方法来估计下降的纵向或横断面生物监测数据所指示的暴露减少程度。根据本文中提出的评估方法,PFOA在人体中的固有消除半衰期为2.4年,代表独立估计的平均值2.5年(95% CI,2.4-2.7)和2.3年(95% CI,2.1-2.4)。1999-2008年期间,美国一般成年人口血液中PFOA浓度的下降代表估计的暴露减少20-30%。
Determination of the chemical clearance rate from human blood is a critical component of toxicokinetic exposure assessment. Analysis of temporal biomonitoring data without consideration of ongoing exposure results in calculation of apparent elimination half-life values that are longer than the intrinsic value. The intrinsic elimination half-life is solely a function of the rate of elimination while the apparent elimination half-life reflects the processes of both elimination and ongoing exposure. Confusion between intrinsic and apparent half-life values can lead to misinterpretation of biomonitoring data and can result in exaggerated predictions in subsequent modeling efforts. This work provides a review of the first-order equations that have been developed to calculate intrinsic and apparent half-life values and the potential bias that can result from confusing these two values. Published human biomonitoring data forperfluorooctanoic acid (PFOA) are analyzed using these equations to provide examples of low, medium and high bias in determination of the intrinsic elimination half-life from plasma or serum, the components of blood typically analyzed for PFOA. An approach is also provided to estimate the extent of exposure reduction that is indicated by declining longitudinal or cross-sectional biomonitoring data. Based on the evaluation methodology presented in this work, the intrinsic elimination half-life of PFOA in humans is 2.4 years, representing the average of independent estimates of 2.5 years (95% CI, 2.4-2.7) and 2.3 years (95% CI, 2.1-2.4). The declining concentration of PFOA in blood of the general USA adult population represents an estimated exposure reduction of 20-30% over the period 1999-2008.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
全氟烷基酸(PFAAs)已在背景人群的血清中以低浓度被检测到。与女性相比,成年男性的浓度更高,可能的解释是月经为女性提供了一个额外的消除途径。在这项研究中,我们检查了血液流失作为PFAAs消除途径的重要性。从进行持续血液抽吸的医疗程序的个体收集了合并的血清样本,该程序称为静脉切开术。静脉切开术患者的男性全氟己烷磺酸(PFHxS)、全氟辛烷磺酸(PFOS)和全氟辛酸(PFOA)的浓度比普通人群中的男性低大约40%。一个简单的药代动力学模型被用来检验血液流失是否可以解释为什么成年男性的PFAAs浓度比女性高,以及为什么接受静脉切开术的男性比普通人群中的男性浓度低。模型应用一般支持这些假设,显示静脉切开术可能会使血液血清浓度降低37%(PFOA)和53%(PFOS),与观察到的44%和37%的差异相比。月经被建模显示,与背景人群中男性和女性之间24%的浓度差异相比,PFOA血清浓度降低了22%。模型和数据的不确定性被识别并讨论。
Perfluorinated alkyl acids (PFAAs) have been detected in serum at low concentrations in background populations. Higher concentrations have been observed in adult males compared to females, with a possible explanation that menstruation offers females an additional elimination route. In this study, we examined the significance of blood loss as an elimination route of PFAAs. Pooled serum samples were collected from individuals undergoing a medical procedure involving ongoing blood withdrawal called venesection. Concentrations from male venesection patients were approximately 40% lower than males in the general population for perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). A simple pharmacokinetic model was used to test the hypothesis that blood loss could explain why adult males have higher concentrations of PFAAs than females, and why males undergoing venesections had lower concentrations compared to males in the general population. The model application generally supported these hypotheses showing that venesection might reduce blood serum concentrations by 37% (PFOA) and 53% (PFOS) compared to the observed difference of 44% and 37%. Menstruation was modeled to show a 22% reduction in PFOA serum concentrations compared to a 24% difference in concentrations between males and females in the background population. Uncertainties in the modeling and the data are identified and discussed.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在这项研究中,在中国天津的普通成人和孕妇中分析了81份全血-尿液配对样本中的PFOS和PFOA。PFOS和PFOA在成人尿液(AU)样本中的检出率分别为48%和76%,几何平均(GM)浓度分别为0.011和0.008 ng/mL;而在母体尿液(MU)样本中PFOS和PFOA的浓度相对较低,GM浓度分别为0.006和0.003 ng/mL。对于PFOA,全血浓度与肌酐调整和不调整尿液的浓度之间的皮尔逊相关系数分别为0.348(p=0.013)和0.417(p=0.002)。成人的PFOS(PFOSUER)和PFOA(PFOAUER)的尿液排泄率分别为16%和25%。这些结果表明尿液是全氟烷基物质(PFASs)排泄的重要途径。孕妇中PFAS浓度在尿液与全血之间(PFASU/B)的分配比率(PFOSU/B,0.0004;PFOAU/B,0.0011)显著低于非孕妇(PFOSU/B,0.0013;PFOAU/B,0.0028)的比率(p=0.025对于PFOSU/B,p=0.017对于PFOAU/B)。此外,我们的结果提示PFOA的尿液排泄存在明显的性别差异,男性成人(31%)的PFOAUER显著高于女性成人(19%)。PFOSUER与年龄显著负相关(r=-0.334,p=0.015);这些发现表明PFOS的尿液排泄在年轻人中比老年人更快。
... In this study, PFOS and PFOA were analyzed in 81 whole blood-urine paired samples from general adults and pregnant women in Tianjin, China. PFOS and PFOA were detected in 48 and 76% of adult urine (AU) samples, with geometric mean (GM) concentrations of 0.011 and 0.008 ng/mL, respectively; whereas relatively low PFOS and PFOA concentrations were found in maternal urine (MU) samples, with GM concentrations of 0.006 and 0.003 ng/mL, respectively. For PFOA, the coefficients of Pearson's correlation between whole blood concentrations and creatinine-adjusted and creatinine-unadjusted urinary concentrations were 0.348 (p=0.013) and 0.417 (p=0.002), respectively. The GM urinary elimination rates of PFOS (PFOSUER) and PFOA (PFOAUER) were 16 and 25%, respectively, for adults. These results indicate that urine is an important pathway of excretion of perfluoroalkyl substances (PFASs). The partitioning ratios of PFAS concentration between urine and whole blood (PFASU/B) in pregnant women (PFOSU/B, 0.0004; PFOAU/B, 0.0011) were significantly lower (p=0.025 for PFOSU/B, p=0.017 for PFOAU/B) than the ratios found in non-pregnant women (PFOSU/B, 0.0013; PFOAU/B, 0.0028). Furthermore, our results suggest a clear gender difference in the urinary elimination of PFOA, with male adults (31%) having significantly higher PFOAUER than that of female adults (19%). PFOSUER was significantly inversely correlated with age (r=-0.334, p=0.015); these findings suggest that urinary elimination of PFOS is faster in young adults than in the elderly.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:T
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S36/37,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:2
-
海关编码:29159080
-
危险品运输编号:UN 3261 8/PG 3
-
RTECS号:RH0781000
-
包装等级:III
-
危险类别:8
-
危险标志:GHS05,GHS07,GHS08
-
危险性描述:H302 + H332,H318,H351,H360D,H362,H372
-
危险性防范说明:P201,P260,P263,P280,P305 + P351 + P338 + P310,P308 + P313
-
储存条件:本品应密封保存,并储放在干燥、清洁、避光的地方,室温不宜超过40℃。运输时要避免强烈震动,堆放时不能倒置。 宜贮存于玻璃或耐腐蚀合金不锈钢和铝容器中,确保严密封存。建议将储存于阴凉、干燥、通风的库房内,并远离热源,防晒防潮。
SDS
模块 1. 化学品
1.1 产品标识符
: 全氟辛酸
产品名称
1.2 鉴别的其他方法
PentadECafluorooctanoic acid
Perfluorocaprylic acid
Perfluorooctanoic acid
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
皮肤腐蚀 (类别 1B)
严重眼睛损伤 (类别 1)
急性水生毒性 (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H302 吞咽有害。
H314 造成严重皮肤灼伤和眼损伤。
H402 对水生生物有害。
警告申明
预防措施
P260 不要吸入粉尘或烟雾。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P273 避免释放到环境中。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P301 + P330 + P331 如果吞咽:漱口,不要催吐。
P303 + P361 + P353 如果皮肤(或头发)接触:立即除去/脱掉所有沾污的衣物,用水清洗皮肤/淋
浴。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P363 沾污的衣服清洗后方可再用。
安全储存
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: PentadECafluorooctanoic acid
别名
Perfluorocaprylic acid
Perfluorooctanoic acid
: C8HF15O2
分子式
: 414.07 g/mol
分子量
组分 浓度或浓度范围
PentadECafluorooctanoic acid
<=100%
化学文摘登记号(CAS 335-67-1
No.) 206-397-9
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
立即脱掉被污染的衣服和鞋。 用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
咳嗽, 呼吸短促, 头痛, 恶心, 呕吐
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氟化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 薄片
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
2.6 在 1 g/l
e) 熔点/凝固点
熔点/凝固点: 55 - 56 °C - lit.
f) 沸点、初沸点和沸程
189 °C 在 981 hPa - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
0.69 hPa 在 25 °C
l) 蒸汽密度
无数据资料
m) 密度/相对密度
0.900 g/cm3
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 腹膜内的 - 大鼠 - 189 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
细胞突变性-体内试验 - 大鼠 - 经口
DNA损伤
细胞突变性-体内试验 - 大鼠 - 腹膜内的
DNA损伤
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 该物质对组织、粘膜和上呼吸道破坏力强
摄入 误吞对人体有害。 引致灼伤。
皮肤 通过皮肤吸收可能有害。 引起皮肤灼伤。
眼睛 引起眼睛灼伤。
接触后的征兆和症状
咳嗽, 呼吸短促, 头痛, 恶心, 呕吐
附加说明
化学物质毒性作用登记: RH0781000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
对水生生物有害。
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 3261 国际海运危规: 3261 国际空运危规: 3261
14.2 联合国运输名称
欧洲陆运危规: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (PentadECafluorooctanoic acid)
国际海运危规: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (PentadECafluorooctanoic acid)
国际空运危规: CorrOSiVE solid, acidic, organic, n.o.s. (PentadECafluorooctanoic acid)
14.3 运输危险类别
欧洲陆运危规: 8 国际海运危规: 8 国际空运危规: 8
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
全氟辛酸概述
全氟辛酸中的C-F键键能极大(486KJ/mol),非常稳定,是自然界中较难破坏的化学键之一。即使在强酸、强碱、高温及强氧化剂的作用下也无法使它断裂。
法规规定2020年6月15日,欧盟在其官方公报发布了修订指令(EU) 2020/784,修订了欧盟新持久性有机污染物法规(EU) 2019/1021的附件I,增加了全氟辛酸(PFOA)及其盐和相关物质的要求,并于2020年7月4日生效。此外,欧盟委员会计划将PFOA及其盐和相关物质从REACH法规限制篇物质清单中删除。
毒性 肝脏毒性全氟辛酸(PFOA)由于其结构特点不易与油脂结合,主要与蛋白质结合,并在肝脏和血液中富集。它会降低抗氧化酶的活性,对肝脏造成损伤。研究显示,将0.1g/kg的PFOA注入小鼠腹腔后,3天内肝脏就会肿大。
生殖和发育毒性全氟辛酸会破坏动物的生殖系统,导致生殖腺萎缩、产卵率下降以及后代发育迟缓等一系列问题。
其他损伤- 抑制免疫系统功能;
- 影响线粒体代谢;
- 改变基因表达;
- 干扰酶活性;
- 破坏细胞膜结构;
- 扰乱甲状腺功能等。
全氟辛酸是一种白色晶体,在32℃水中的溶解度为0.01~0.023mol/L。
用途 主要用途 其他用途全氟辛酸及其衍生物被用作高效表面活性剂、分散剂和添加剂,主要用于合成含氟憎水憎油剂、皮革整理剂等产品。
生产方法将纯度为99.5%的辛酰氯与氟化氢及少量正丁基硫酸投入电解槽,在20-25℃下通电(电压5-8V),电解产物用碱中和,再用酸酸化,蒸馏即可得到全氟辛酸。原料消耗定额为:辛酰氯6000-8000kg/t、氢氟酸12000kg/t、硫酸1500kg/t、氢氧化钠1500kg/t。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 全氟壬酸 perfluorononanoic acid 375-95-1 C9HF17O2 464.079 十五氟辛酸甲酯 methyl perfluorooctanoate 376-27-2 C9H3F15O2 428.098 —— bis(pentadecafluoro-octanoyl) peroxide 34434-27-0 C16F30O4 826.126 —— perfluorononanal 63967-40-8 C9HF17O 448.079 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-十五氟辛-1-醇 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluoro-1-octanol 307-30-2 C8H3F15O 400.087 —— phenyl perfluorooctanoate —— C14H5F15O2 490.169 全氟辛氟 perfluorooctanoyl fluoride 335-66-0 C8F16O 416.062 —— p-nitrophenyl perfluoroctanoate —— C14H4F15NO4 535.166 全氟辛酰氯 pentadecafluorooctanoyl chloride 335-64-8 C8ClF15O 432.516 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— [18F] perfluorooctanoic acid —— C8HF15O2 399.095 全氟庚酸 perfluoroheptanoic acid 375-85-9 C7HF13O2 364.063 全氟己酸 undecafluorohexanoic acid 307-24-4 C6HF11O2 314.055 十五氟辛酸甲酯 methyl perfluorooctanoate 376-27-2 C9H3F15O2 428.098 九氟戊酸 Perfluoropentanoic acid 2706-90-3 C5HF9O2 264.047 全氟辛酸乙酯 ethyl perfluorooctanoate 3108-24-5 C10H5F15O2 442.125 全氟辛酸正丁酯 pentadecafluoro-octanoic acid butyl ester 307-96-0 C12H9F15O2 470.178 —— pentadecafluorooctanoate d'heptyle 127392-52-3 C15H15F15O2 512.259 —— 1,6-bis-pentadecafluorooctanoyloxy-hexane 559-08-0 C22H12F30O4 910.287 —— isopropyl 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctanoate 106608-82-6 C11H7F15O2 456.151 —— hydro-2 perfluorooctanoate d'heptyle 118663-63-1 C15H16F14O2 494.268 —— bis(pentadecafluoro-octanoyl) peroxide 34434-27-0 C16F30O4 826.126 —— C7F15CO2Si(CH3)3 64616-93-9 C11H9F15O2Si 486.253 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-十五氟辛-1-醇 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluoro-1-octanol 307-30-2 C8H3F15O 400.087 —— 2-methacryloyloxyethyl perfluorooctanoate 14814-77-8 C14H9F15O4 526.199 —— dihydro-2,2 perfluorooctanoate d'heptyle 127392-57-8 C15H17F13O2 476.278 —— benzyl perfluoroocatanoate 208663-34-7 C15H7F15O2 504.195 —— methylacryloyloxyl-2-hydroxypropyl perfluorooctanoate 34569-65-8 C15H11F15O5 556.225 全氟辛氟 perfluorooctanoyl fluoride 335-66-0 C8F16O 416.062 —— 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctan-1,1-diol 31185-69-0 C8H3F15O2 416.087 —— 1-(perfluoro n-heptyl) ethanol-(1) 24015-83-6 C9H5F15O 414.114 1H,1H,1H-十五氟-2-壬酮 3,3,4,4,5,5,6,6,7,7,8,8,9,9,9-pentadecafluorononan-2-one 754-85-8 C9H3F15O 412.098 —— 1,1,1,2,2,3,3,4,4,5,5,6,6,7,7-Pentadecafluoro-hexadecan-8-one 41049-15-4 C16H17F15O 510.286 烯丙基1H,1H-全氟辛基醚 8-(allyloxyl)-1,1,1,2,2,3,3,4,4,5,5,6,6,7,7-pentadecafluorooctane 812-72-6 C11H7F15O 440.152 9H,9H-全氟-8,10-十七碳烷二酮 1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,11,11,12,12,13,13,14,14,15,15,16,16,17,17,17-triacontafluoroheptadecan-8,10-dione 36554-97-9 C17H2F30O2 808.154 七氟丁酸 heptafluorobutyric Acid 375-22-4 C4HF7O2 214.04 —— trichloro-3-(1H,1H-pentadecafluorooctyloxy)propylsilane 755-08-8 C11H8Cl3F15OSi 575.604 —— 2-methyl-3,3,4,4,5,5,6,6,7,7,8,8,9,9,9-pentadecafluorononan-2-ol 92914-88-0 C10H7F15O 428.141 —— Bis-<Ψ'-octyl>-3-methylglutarat 647-39-2 C22H12F30O4 910.287 全氟辛酰氯 pentadecafluorooctanoyl chloride 335-64-8 C8ClF15O 432.516 二氯异氰尿酸纳 perfluorooctanoyl ammonia 423-54-1 C8H2F15NO 413.086 十三氟庚酰胺 tridecafluoroheptamide 2358-22-7 C7H2F13NO 363.078 —— α-Methylphenacyl perflurooctanoate 81559-39-9 C17H9F15O3 546.233 —— 1H,1H,1H,2H,3H-pentadecafluoro-undec-2t-en-4-one 2059-16-7 C11H5F15O 438.136 —— dimethyl 2,3-bis(2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)succinate 53392-98-6 C22H12F30O4 910.287 —— cholesteryl perfluorooctanoate —— C35H45F15O2 782.717 全氟十四烷 perfluorotetradecane 307-62-0 C14F30 738.106 —— perfluoro-1H-heptane 375-83-7 C7HF15 370.061 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:Zur herstellung von Perfluoralkylisocyanaten und 1,1-Dihydroperfluoralkylisocyanaten摘要:DOI:10.1007/bf00901328
-
作为产物:参考文献:名称:一种制备全氟羧酸的新方法摘要:伯全氟烷基碘化物和含3到12个碳原子的溴化物[CF 3(CF 2)n X,n = 2 -11,X = Br,I]与Rongalite-NaHCO 3试剂在偶极非质子传递溶剂中的反应,例如DMF或DMSO,已经过调查。反应以51-86%的收率得到全氟羧酸钠[CF 3(CF 2)n-1 CO 2 Na,n = 2〜11],并将它们转化为相应的全氟羧酸[CF 3(CF 2)n- 1 CO 2H,n = 2 11]通过硫酸处理。这提供了合成全氟羧酸的新方法。DOI:10.1016/s0022-1139(00)82053-9
-
作为试剂:参考文献:名称:Regioselective C–H hydroxylation of n-alkanes using Shilov-type Pt catalysis in perfluorinated micro-emulsions摘要:在这项工作中,展示了将Shilov型PtII和胶束催化相结合,利用水作为反应介质实现对饱和的
n -烷烃进行挑战性的末端C-H羟基化的潜力。DOI:10.1039/c9cy02320h
文献信息
-
Direct ester condensation from a 1:1 mixture of carboxylic acids and alcohols catalyzed by hafnium(IV) or zirconium(IV) salts作者:Kazuaki Ishihara、Masaya Nakayama、Suguru Ohara、Hisashi YamamotoDOI:10.1016/s0040-4020(02)00966-3日期:2002.10either carboxylic acids or alcohols are normally needed. We found that the direct condensation of equimolar amounts of carboxylic acids and alcohols could be achieved using hafnium(IV) or zirconium(IV) salts. These metal salts are highly effective as catalysts for the selective esterification of primary alcohols with carboxylic acids in the presence of secondary alcohols or aromatic alcohols. The present
-
An Efficient, Overall [4+1] Cycloadditon of 1,3-Dienes and Nitrene Precursors作者:Qiong Wu、Jian Hu、Xinfeng Ren、Jianrong Steve ZhouDOI:10.1002/chem.201101630日期:2011.10.4Intermolecular cycloadditions of conjugated dienes and nitrene precursors usually produce aziridines. A generally useful method was lacking to directly provide the [4+1] cycloadducts, 3‐pyrrolines. We have realized this transformation by using an uniquely active catalyst, copper(II) 1,1,1,5,5,5‐hexafluoroacetylacetonate ([Cu(hfacac)2]). The method is applicable to a wide array of dienes with good yields
-
[EN] CRYSTALLINE AND LIQUID CRYSTALLINE 25-HYDROXY-CHOLEST-5-EN-3-SULFATE SODIUM AND METHODS FOR PREPARING SAME<br/>[FR] 25-HYDROXY-CHOLEST-5-EN-3-SULFATE SODIQUE CRISTALLIN ET CRISTALLIN LIQUIDE ET SES PROCÉDÉS DE PRÉPARATION申请人:DURECT CORP公开号:WO2021133976A1公开(公告)日:2021-07-01Crystalline and liquid crystalline forms of 25HC3S sodium are described herein. The disclosure includes Forms I, II, III, V, IX, XI, and XIII of 25HC3S sodium and combinations thereof. Pharmaceutical formulations of said forms, or combinations thereof, and methods of treating or preventing disease such as hypercholesterolemia, hypertriglyceridemia, and conditions related to fat accumulation and inflammation (e.g., non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), alcoholic hepatitis, acute kidney injury (AKI), psoriasis, and atherosclerosis) are further disclosed herein. Methods for preparing 25HC3S are also provided
-
[EN] QUINOLIN-4-ONE AND 4(1H)-CINNOLINONE COMPOUNDS AND METHODS OF USING SAME<br/>[FR] COMPOSÉS DE QUINOLIN-4-ONE ET DE 4(1H)-CINNOLINONE ET PROCÉDÉS D'UTILISATION ASSOCIÉS申请人:FREQUENCY THERAPEUTICS INC公开号:WO2020163816A1公开(公告)日:2020-08-13The present disclosure relates to quinolin-4-one and 4(1H)-cinnolinone compounds and methods of using them to induce self-renewal of stem/progenitor supporting cells, including inducing the stem/progenitor cells to proliferate while maintaining, in the daughter cells, the capacity to differentiate into tissue cells.
-
Perfluoroalkylation of Olefins by Electrooxidation of Perfluoroalkanoic Acids: Relations between Product-Selectivity and Current Density and Structures of Olefins作者:Kenji Uneyama、Shunsuke Watanabe、Yukio Tokunaga、Kouichi Kitagawa、Yasuhiro SatoDOI:10.1246/bcsj.65.1976日期:1992.7Electrooxidation of perfluoroalkanoic acids RfCO2H (Rf = CF3, C3F7, C7F15 together with CHF2, CH2F) in the presence of electron-deficient olefins (methyl acrylate, methyl methacrylate, acrylamide, and acrylonitrile) provided perfluoroalkylated products. The electrolysis was conducted in MeCN–H2O (7 : 1) using platinum electrodes in an undivided cell. Dimerization of methyl acrylate accompanying perfluoroalkylation在缺电子烯烃(丙烯酸甲酯、甲基丙烯酸甲酯、丙烯酰胺和丙烯腈)存在下,全氟链烷酸 RfCO2H(Rf = CF3、C3F7、C7F15 以及 CHF2、CH2F)的电氧化得到全氟烷基化产物。电解是在 MeCN-H2O (7:1) 中使用铂电极在未分隔的电池中进行的。伴随全氟烷基化的丙烯酸甲酯二聚反应在 20 mA cm-2 电流密度下以 40-50% 的产率发生。在高电流密度(100-200 mA cm-2)下,丙烯酰胺中碳-碳双键的 1,2-加成占主导地位。另一方面,三氟甲基和乙酰氨基的1,2-加成以及三氟甲基和羟基的1,2-加成发生在甲基丙烯酸甲酯的低电流密度(<5 mA cm-2)电解中。
表征谱图
-
氢谱1HNMR
-
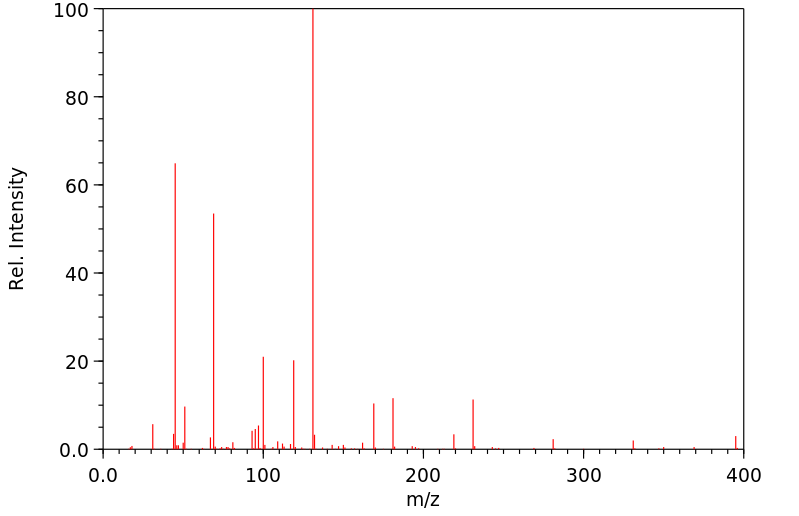
质谱MS
-
碳谱13CNMR
-
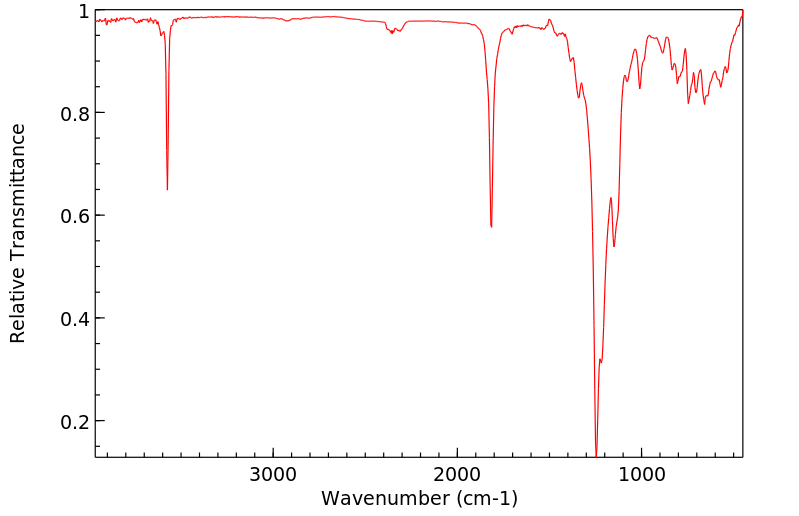
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-氯环己基高氯酸盐
顺式-1-溴-2-氟-环己烷
顺式-1-叔丁基-4-氯环己烷
顺式-1,2-二氯环己烷
顺-1H,4H-十二氟环庚烷
镓,三(三氟甲基)-
镁二(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-十七氟-1-辛烷磺酸酯)
铵2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-二十三氟十二烷酸盐
铜N-(2-氨基乙基)乙烷-1,2-二胺2-氰基胍二氯化盐酸
钾{[(十七氟辛基)磺酰基](甲基)氨基}乙酸酯
钠3-[(3-{[(十七氟辛基)磺酰基]氨基}丙基)(甲基)氨基]-1-丙烷磺酸酯
重氮基烯,(1-溴环己基)(1,1-二甲基乙基)-,1-氧化
辛酸,十五氟-,2-(1-羰基辛基)酰肼
赖氨酰-精氨酰-精氨酰-苯基丙氨酰-赖氨酰-赖氨酸
诱蝇羧酯B1
诱蝇羧酯
萘并[2,1-b]噻吩-1(2H)-酮
膦基硫杂酰胺,P,P-二(三氟甲基)-
脲,N-(4,5-二甲基-4H-吡唑-3-基)-
肼,(3-环戊基丙基)-,盐酸(1:1)
组织蛋白酶R
磷亚胺三氯化,(三氯甲基)-
碳标记全氟辛酸
碘甲烷与1-氮杂双环(4.2.0)辛烷高聚合物的化合物
碘甲烷-d2
碘甲烷-d1
碘甲烷-13C,d3
碘甲烷
碘环己烷
碘仿-d
碘仿
碘乙烷-D1
碘[三(三氟甲基)]锗烷
硫氰酸三氯甲基酯
甲烷,三氯氟-,水合物
甲次磺酰胺,N,N-二乙基-1,1,1-三氟-
甲次磺酰氯,氯二[(三氟甲基)硫代]-
甲基碘-12C
甲基溴-D1
甲基十一氟环己烷
甲基丙烯酸正乙基全氟辛烷磺
甲基三(三氟甲基)锗烷
甲基[二(三氟甲基)]磷烷
甲基1-氟环己甲酸酯
环戊-1-烯-1-基全氟丁烷-1-磺酸酯
环己烷甲酸4,4-二氟-1-羟基乙酯
环己烷,1-氟-2-碘-1-甲基-,(1R,2R)-rel-
环己基五氟丙烷酸酯
环己基(1-氟环己基)甲酮
烯丙基十七氟壬酸酯