代谢
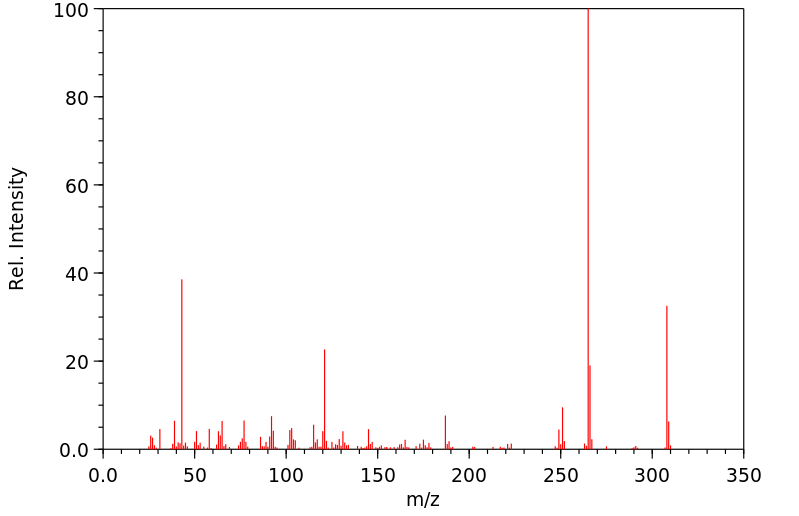
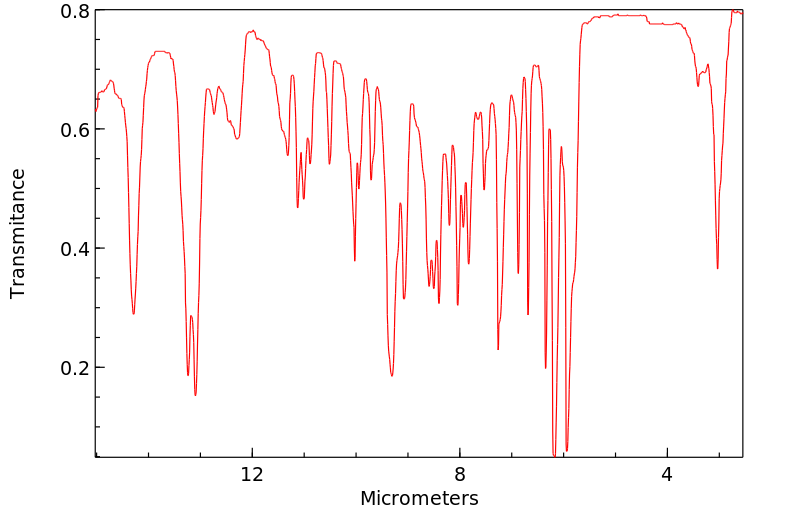
华法林的代谢是立体选择性和区域选择性的。[A2460]主要的代谢途径是氧化成各种羟基华法林,占总代谢物的80-85%。CYP2C9是主要的酶,催化S-华法林的6-和7-羟基化,而4'-羟基化是通过CYP2C18实现的,CYP2C19也有少量贡献。R-华法林通过CYP2C8代谢成4'-羟基华法林,CYP2C19也有一定贡献,6-和8-羟基华法林通过CYP1A2和CYP2C19代谢,7-羟基华法林通过CYP1A2和CYP2C8代谢,最后通过CYP3A4代谢成10-羟基华法林。10-羟基华法林代谢物以及一种苄醇代谢物经历一个消除步骤形成脱氢华法林。代谢的次要途径是将酮羰基还原成华法林醇,占总代谢物的20%。与硫酸盐和葡萄糖酸基团有限的结合反应发生,但这些代谢物只被证实存在于R-羟基华法林中。
Metabolism of warfarin is both stereo- and regio-selective.[A2460] The major metabolic pathway is oxidation to various hydroxywarfarins, comprising 80-85% of the total metabolites. CYP2C9 is the major enzyme catalyzing the 6- and 7-hydroxylation of S-warfarin while 4'-hydroxylation occurs through CYP2C18 with minor contributions from CYP2C19. R-warfarin is metabolized to 4'-hydroxywarfarin by CYP2C8 with some contirbuting by CYP2C19, 6- and 8-hydroxywarfarin by CYP1A2 and CYP2C19, 7-hydroxywarfarin by CYP1A2 and CYP2C8, and lastly to 10-hydroxywarfarin by CYP3A4. The 10-hydroxywarfarin metabolite as well as a benzylic alcohol metabolite undergo an elimination step to form dehydrowarfarin. The minor pathway of metabolism is the reduction of the ketone group to warfarin alcohols, comprising 20% of the metabolites. Limited conjugation occurs with sulfate and gluronic acid groups but these metabolites have only been confirmed for R-hydroxywarfarins.
来源:DrugBank