2,4-二氯酚 | 120-83-2
中文名称
2,4-二氯酚
中文别名
2,4-二氯苯酚;2.4二氯酚;2, 4-二氯苯酚;2,4-双氯酚
英文名称
2,4-dichlorophenol
英文别名
2,4-DCP;DCP
CAS
120-83-2
化学式
C6H4Cl2O
mdl
——
分子量
163.003
InChiKey
HFZWRUODUSTPEG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:42-43 °C(lit.)
-
沸点:209-210 °C(lit.)
-
密度:1.383
-
闪点:237 °F
-
溶解度:可溶于甲醇:1g/10ml
-
LogP:3.25 at 20℃
-
物理描述:2,4-dichlorophenol is a colorless crystalline solid with a medicinal odor. Melting point 45°C. Sinks in water. Strong irritant to tissues; toxic by ingestion.
-
颜色/状态:COLORLESS CRYSTALS OR NEEDLES
-
气味:Strong medicinal
-
蒸汽密度:5.62 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.09 mm Hg at 25 °C
-
大气OH速率常数:1.06e-12 cm3/molecule*sec
-
自燃温度:500 °C
-
分解:When heated to decomposition ... it emits highly toxic fumes of /hydrogen chloride/.
-
汽化热:13,230.4 cal/mole
-
气味阈值:Odor detection in water: 2.10X10-1 ppm (chemically pure)
-
解离常数:pKa = 7.89
-
保留指数:1144.8;1148;1151;1147;1158;1168;1170;1165;1160;1170;1181;1183;1170;1160;1183;1138;1143;1144;1192.9;1132.2;1140;1140
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
代谢
2,4-Dichlorophenol is excreted as a conjugate of glucuronic acid. up to 16% may be excreted as sulfate in urine of rabbits.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Mice were given (14)C labeled gamma- or beta-benzene hexachloride by intraperitoneal injection, and the appearance of metabolites in the urine was monitored. 2,4-DCP and 2,4-DCP conjugates were found and identified primarily as glucuronides and sulfates.
来源:Hazardous Substances Data Bank (HSDB)
代谢
(14)C标记的2,4-二氯酚在大鼠离体灌注肝脏中的摄取和代谢被研究。在存在ATP的情况下,肝脏中放射性物质的摄取增加了6.6%,在存在ATP和半乳糖胺的情况下增加了14.9%。这种放射性物质摄取的增加表明维持了肝细胞的完整性。胆汁中的2,4-DCP葡萄糖苷酸结合物通过过甲基化衍生并经气相色谱-质谱鉴定。通过用己烷提取,从肝脏和灌注液中分离出两种不寻常的代谢物。这些代谢物的气相色谱图在保留时间12.0和15.5分钟处给出峰,并通过质谱鉴定。这些代谢物的裂解模式确认它们的身份为二氯甲氧基酚。
The uptake and metabolism of (14)C-labeled 2,4-dichlorophenol was studied in the isolated perfused rat liver. The uptake of radioactivity in liver increased 6.6% in the presence of ATP and 14.9% in the presence of ATP and galactosamine. This increase in the uptake of radioactivity was indicative of maintaining the integrity of liver cells. The glucuronide conjugate of 2,4-DCP in bile was derivatized by permethylation and characterized by gas chromatography-mass spectrometry. Two unusual metabolites were isolated from the liver and perfusate by extraction with hexane. The gas chromatogram of these metabolites gave peaks at the retention times 12.0 and 15.5 min, and were characterized by mass spectrometry. The fragmentation pattern of these metabolites confirmed their identity as dichloromethyoxyphenols.
来源:Hazardous Substances Data Bank (HSDB)
代谢
... Various chlorophenols /including 2,4-dichlorophenol/ are formed as intermediate metabolites during the microbiological degradation of the herbicides 2,4-D & 2,4,5-T and pesticides Silvex, Ronnel, lindane and benzene hexachloride. /Chlorophenols/
来源:Hazardous Substances Data Bank (HSDB)
代谢
在吸入和皮肤途径之后,2,4-DCP会被迅速吸收并排出体外。关于口服途径吸收的数据仅限于动物研究。当以静脉注射方式给予大鼠时,2,4-DCP会迅速分布到肾脏、肝脏、脂肪和大脑中,其中肾脏和肝脏的浓度最高。2,4-DCP会强烈结合血清蛋白,包括白蛋白和球蛋白。人类和动物研究都表明,硫酸化和葡萄糖苷酸化是氯酚的主要代谢途径。2,4-DCP已被证明可以通过表达人类细胞色素P-450 3A4的酵母Saccharomyces cerevisiae的微粒体部分和整个细胞代谢为两种主要代谢物,被鉴定为2-氯-1,4-羟基醌和2-氯-1,4-苯醌。另一种代谢物,1,2,4-羟基苯,在整体细胞生物转化过程中也被检测到,但在微粒体部分中未观察到。(L159)
After inhalation and dermal routes, 2,4-DCP is rapidly absorbed and excreted. Data on absorption following oral route are limited to animal studies. When administered intravenously to rats, 2,4-DCP rapidly distributes to the kidney, liver, fat, and brain, with the highest concentrations in the kidney and liver. 2,4-DCP strongly bind to serum proteins, including albumin and globulin. Both human and animal studies indicate that sulfation and glucuronidation are the main metabolic pathways of chlorophenols. 2,4-DCP has been shown to be metabolized into two major metabolites identified as 2-chloro-1,4-hydroxyquinone and 2-chloro-1,4-benzoquinone by microsomal fractions and whole cells of yeast Saccharomyces cerevisiae expressing human cytochrome P-450 3A4. Another metabolite, 1,2,4-hydroxybenzene, was also detected during biotransformation by whole cells but was not observed in microsomal fractions. (L159)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
氯酚具有中等偏高的脂溶性。通过胃肠道的吸收是通过简单扩散,预计会既快速又几乎完全。氯酚在皮肤接触后也容易被吸收。氯酚能解偶线粒体的氧化磷酸化并产生惊厥。在低浓度下,解偶联会增加状态4(静息状态)的呼吸作用,这是由于在没有磷酸盐受体的情况下增加了三磷酸腺苷酶(ATP酶)的活性。状态3(活动状态)的呼吸抑制作用也被观察到。在中等浓度下,静息呼吸既不被刺激也不被抑制。在非常高的浓度下,会出现显著的呼吸抑制作用,伴随着电子传递过程的破坏和ATP酶活性的降低。
Chlorophenols have moderately high lipophilicity. Absorption through the gastrointestinal tract is by simple diffusion and is expected to be both rapid and virtually complete. The chlorophenols are also readily absorbed after dermal exposure. Chlorophenols uncouple mitochondrial oxidative phosphorylation and produce convulsions. At low concentrations, uncoupling produces stimulation of state 4 (resting state) respiration as a result of increased adenosine triphosphatase (ATPase) activity in the absence of a phosphate acceptor. Inhibition of state 3 (active) respiration is also observed. At moderate concentrations, resting respiration is neither stimulated nor inhibited. Significant inhibition of respiration, associated with a breakdown of the electron transport process and decreased ATPase activity, occurs at very high concentrations. (L159)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
评价:对于人类而言,对于多氯酚或其钠盐的联合暴露的致癌性证据有限。有证据表明,在实验动物中2,4-二氯酚不具有致癌性。...总体评价:多氯酚或其钠盐的联合暴露对人类可能是致癌的(2B组)。/多氯酚及其钠盐/
Evaluation: There is limited evidence in humans for the carcinogenicity of combined exposures to polychlorophenols or to their sodium salts. There is evidence suggesting lack of carcinogenicity of 2,4-dichlorophenol in experimental animals. ... Overall evaluation: Combined exposures to polychlorophenols or to their sodium salts are possibly carcinogenic to humans (Group 2B). /Polychlorophenols and their sodium salts/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
2B,可能对人类有致癌性。
2B, possibly carcinogenic to humans. (L135)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
氯酚通过皮肤吸收的剂量可能比口服吸收的剂量更有毒性。一名工人在意外被2,4-DCP(2,4-二氯苯酚)溅到右臂和腿上后,20分钟内经历了癫痫发作,倒下,并在不久后死亡。在接触氯酚的动物中,已经报告了昏睡、震颤、惊厥和/或中枢神经系统抑制的情况。
Dermally absorbed doses of chlorophenols are potentially more toxic than orally absorbed doses. Within 20 minutes of being accidentally splashed with 2,4-DCP on his right arm and leg, a worker experienced seizures, collapsed, and died shortly thereafter. Lethargy, tremors, convulsions, and/or central nervous system depression have been reported in chlorophenol-exposed animals. (L159)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
该物质可通过吸入、皮肤接触和摄入被人体吸收。所有接触途径都会产生严重的局部影响。
The substance can be absorbed into the body by inhalation, through the skin and by ingestion. Serious local effects by all routes of exposure.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
... Readily absorbed from gastrointestinal tract and from parenteral sites of injection. /Chlorophenols/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
2,4-二氯酚(2,4-DCP)在大鼠体内的代谢和分布经过静脉注射10 mg/kg后进行了研究。发现肾脏中的浓度最高,其次是肝脏、脂肪和大脑。2,4-DCP被代谢成葡萄糖苷酸和其他结合物。母化合物及其结合物迅速从体内消除。2,4-DCP及其结合物在血浆、脂肪、大脑、肝脏和肾脏中的半衰期范围为4到30分钟。2,4-DCP在血浆中的分布体积为3.7 L/kg。组织/血浆比率表明2,4-DCP对肾脏有更大的亲和力。
The metabolism and distribution of 2,4-dichlorophenol (2,4-DCP) were studied in rat after iv admin of 10 mg/kg. Highest concn was found in kidney, followed by liver, fat, & brain. 2,4-DCP is metabolized to glucuronide & other conjugates. The parent cmpd & its conjugates were rapidly eliminated from body. Half-life of 2,4-DCP & its conjugates in plasma, fat, brain, liver, & kidney ranged from 4 to 30 min. Vol of distribution of 2,4-DCP in plasma was 3.7 L/kg. Tissue/plasma ratios indicated that 2,4-DCP has a greater affinity for kidney.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Because of their high lipid solubility and low ionization at physiological pH, dichlorophenols would be expected to be readily absorbed following ingestion. /Dichlorophenols/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
牛体内吸收的牛尿和脂肪参考材料是通过口服给牛投喂林丹、1,2-二氯苯、2,4-二氯酚、1,2,3,4-四氯苯和五氯酚来制备的。获得的组织和液体经过均质化处理,并对其中的投喂化合物和主要代谢物进行分析。几乎所有的20克剂量的2,4-二氯酚在投喂后24小时内通过尿液排出。...发现尿液样本在冷冻/解冻四个周期后,氯酚浓度保持恒定,因此可以认为是相当稳定的。...
In vivo incorporated bovine urine and adipose reference materials were prepared by oral administration of lindane, 1,2-dichlorobenzene, 2,4-dichlorophenol, 1,2,3,4-tetrachlorobenzene, and pentachlorophenol to cows. The tissues and fluids obtained were homogenized and analyzed for administered compounds and major metabolites. Virtually all of the 20 g dose of 2,4-dichlorophenol was eliminated in the urine within 24 hr following administration. ... The urine samples were found to be reasonably stable with chlorophenol concentrations remaining constant over four freeze/thaw cycles. ...
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S16,S26,S36/37,S36/37/39,S45,S61
-
危险类别码:R22,R51/53,R24,R34
-
WGK Germany:3
-
海关编码:29081000
-
危险品运输编号:UN 2928 6.1/PG 2
-
危险类别:6.1
-
RTECS号:SK8575000
-
包装等级:III
-
储存条件:密封保存于阴凉避光处。 该产品采用铁桶包装。请注意,本品易燃,应远离火源,并储存在阴凉、干燥、通风的地方。若发生2,4-二氯(苯)酚火灾,请使用水、黄沙或二氧化碳泡沫进行灭火。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 2,4-二氯酚 |
| 化学品英文名称: | 2,4-Dichlorophenol |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 120-83-2 |
| 分子式: | C 6 H 4 Cl 2 O |
| 分子量: | 163.00 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:2,4-二氯酚 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 无资料 |
| 健康危害: | 吸入、摄入或经皮肤吸收对身体有害。对眼睛、粘膜、呼吸道及皮肤有刺激作用,重者可引起灼伤。 |
| 环境危害: | 无资料 |
| 燃爆危险: | 本品可燃,有毒,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 立即脱去污染的衣着,用大量流动清水冲洗至少15分钟。就医。 |
| 眼睛接触: | 立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 |
| 食入: | 用水漱口,给饮牛奶或蛋清。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火能燃烧。与氧化剂接触猛烈反应。受热或接触酸或酸雾, 产生氯化物烟气。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 灭火方法及灭火剂: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | 无资料 |
| 禁止使用的灭火剂: | 无资料 |
| 闪点(℃): | 113 |
| 自燃温度(℃): | 无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 无资料 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,提供充分的局部排风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂等分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 无资料 |
| 监测方法: | 4-氨基安替比林分光光度法;溶剂解吸-气相色谱法 |
| 工程控制: | 严加密闭,提供充分的局部排风。提供安全淋浴和洗眼设备。 |
| 呼吸系统防护: | 空气中粉尘浓度超标时,必须佩戴自吸过滤式防尘口罩。紧急事态抢救或撤离时,应该佩戴空气呼吸器。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿防毒物渗透工作服。 |
| 手防护: | 戴橡胶手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作完毕,彻底清洗。单独存放被毒物污染的衣服,洗后备用。注意个人清洁卫生。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色结晶。 |
| pH: | |
| 熔点(℃): | 45 |
| 沸点(℃): | 210 |
| 相对密度(水=1): | 1.38 |
| 相对蒸气密度(空气=1): | 5.62 |
| 饱和蒸气压(kPa): | 0.13/53℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 113 |
| 引燃温度(℃): | 无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 6 H 4 Cl 2 O |
| 分子量: | 163.00 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于水,溶于醇、乙醚、苯、四氯化碳。 |
| 主要用途: | 用于有机合成。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 |
| 禁配物: | 强氧化剂、强酸、酸酐、酰基氯。 |
| 避免接触的条件: | 无资料 |
| 聚合危害: | 无资料 |
| 分解产物: | 无资料 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:580 mg/kg(大鼠经口) LC50:无资料 |
| 急性中毒: | 无资料 |
| 慢性中毒: | 无资料 |
| 亚急性和慢性毒性: | |
| 刺激性: | 无资料 |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | 无资料 |
| 生物降解性: | 在土壤中可生物降解。 |
| 非生物降解性: | 不易光解和水解。 |
| 生物富集或生物积累性: | 陆地上部分被土壤吸附,迁移能力中下,离子态时更易迁移,可生物降解。在水体中可生物降解。 |
| 第十三部分:废弃处置 |
| 废弃物性质: | 无资料 |
| 废弃处置方法: | 用焚烧法处置。与燃料混合后,再焚烧。焚烧炉排出的卤化氢通过酸洗涤器除去。 |
| 废弃注意事项: | 无资料 |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 61704 |
| UN编号: | 无资料 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 塑料袋或二层牛皮纸袋外全开口或中开口钢桶;螺纹口玻璃瓶、铁盖压口玻璃瓶、塑料瓶或金属桶(罐)外普通木箱;螺纹口玻璃瓶、塑料瓶或镀锡薄钢板桶(罐)外满底板花格箱、纤维板箱或胶合板箱。 |
| 运输注意事项: | 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与酸类、氧化剂、食品及食品添加剂混运。运输途中应防曝晒、雨淋,防高温。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定;常用危险化学品的分类及标志 (GB 13690-92)将该物质划为第6.1 类毒害品。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | |
| 填表时间: | 2004年12月9日 |
| 填表部门: | 无资料 |
| 数据审核单位: | 无资料 |
| 修改说明: | 无资料 |
| 其他信息: | |
| MSDS修改日期: | 1900年1月1日 |
制备方法与用途
2,4-二氯酚
用途
2,4-二氯酚又称为2,4-二氯苯酚,是多种农药和医药中间体的重要原料。在农业化学中,它可以用于合成有机磷杀虫剂丙硫磷、除草剂2,4-D(2,4滴)、2,4-滴丁酯、禾草灵、甲缩除草醚、唑啶草酮、丙炔恶草酮以及新杀菌剂氰菌胺等。在医药领域,它可以用作药物硫双二氯酚的重要原料。
制备以苯酚和氯气为原料,混合催化剂由二苯硫醚、三氯化铁、三氟甲磺酸组成,其中二苯硫醚、三氯化铁和三氟甲磺酸的摩尔比为2:2:1。制备高纯度2,4-二氯苯酚的方法如下:
- 将50g苯酚加热融化并搅拌,搅拌速度为2000r/min,按比例加入混合催化剂(占原料苯酚质量的0.05%)。
- 加热物料至70℃,开始缓慢均匀通入氯气,持续3小时,氯气体积为苯酚摩尔数的2.2倍。催化氯化反应6.5小时后得到粗品。
- 将2,4-二氯苯酚粗品加入熔融结晶器内,升温至60℃并恒温维持3小时进行发汗处理,然后以2℃/min的速度缓慢降温至10℃,最终获得高纯度2,4-二氯苯酚产品。
白色固体。有酚味,溶于乙醇、乙醚、氯仿、苯和四氯化碳,微溶于水。
用途主要用作农药和医药中间体,在农药上可以合成有机磷杀虫剂丙硫磷、除草剂2,4-D、2,4-滴丁酯、禾草灵、甲缩除草醚、唑啶草酮(Azafenidin)、丙炔恶草酮(Oxadiargyl)以及新杀菌剂氰菌胺(Fenoxanil)。在医药方面,它是药物硫双二氯酚的重要原料。此外,它还用于有机合成、生化试剂、气相色谱对比样品及植物生长促进剂。
生产方法 类别上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4,6-三氯苯酚 2,4,6-Trichlorophenol 88-06-2 C6H3Cl3O 197.448 邻氯苯酚 2-Chlorophenol 95-57-8 C6H5ClO 128.558 2,4-二氯苯甲醚 1,3-dichloro-4-methoxybenzene 553-82-2 C7H6Cl2O 177.03 三氯酚 2,3,4-trichlorophenol 15950-66-0 C6H3Cl3O 197.448 对氯苯酚 4-chloro-phenol 106-48-9 C6H5ClO 128.558 2,3,4,6-四氯酚 2,3,4,6-tetrachlorophenol 58-90-2 C6H2Cl4O 231.893 五氯酚 Pentachlorophenol 87-86-5 C6HCl5O 266.338 2,4-二氯苯乙醚 2,4-Dichlor-phenetol 5392-86-9 C8H8Cl2O 191.057 —— 2,4-dichlorophenoxymethyl chloride 13543-09-4 C7H5Cl3O 211.475 —— 2,4-dichloro-α-iodoanisole 106262-00-4 C7H5Cl2IO 302.927 —— 1-(allyloxy)-2,4-dichlorobenzene 5441-16-7 C9H8Cl2O 203.068 —— bis(2,4-dichlorophenoxy)methane 35412-53-4 C13H8Cl4O2 338.017 —— (2,4-Dichlor-phenoxy)-trimethyl-silan 17878-30-7 C9H12Cl2OSi 235.185 —— 1-ethoxymethoxy-2,4-dichloro-benzene 829-59-4 C9H10Cl2O2 221.083 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,4,6-三氯苯酚 2,4,6-Trichlorophenol 88-06-2 C6H3Cl3O 197.448 4,6-二氯间苯二酚 4,6-Dichlororesorcinol 137-19-9 C6H4Cl2O2 179.003 邻氯苯酚 2-Chlorophenol 95-57-8 C6H5ClO 128.558 2,4-二氯苯甲醚 1,3-dichloro-4-methoxybenzene 553-82-2 C7H6Cl2O 177.03 2,4,5-三氯苯酚 2,4,5-trichlorophenol 95-95-4 C6H3Cl3O 197.448 3,5-二氯儿茶酚 3,5-dichlorocatechol 13673-92-2 C6H4Cl2O2 179.003 2,5-二氯苯酚 2,5-dichlorophenol 583-78-8 C6H4Cl2O 163.003 对氯苯酚 4-chloro-phenol 106-48-9 C6H5ClO 128.558 2,4-二氯苯乙醚 2,4-Dichlor-phenetol 5392-86-9 C8H8Cl2O 191.057 氯氢醌 Chlorohydroquinone 615-67-8 C6H5ClO2 144.558 2,4-二氯-1-(氟甲氧基)苯 2,4-dichloro-1-(fluoromethoxy)benzene 87453-28-9 C7H5Cl2FO 195.021 7-甲基-7H-嘌呤-6-胺 (2,4-dichloro-phenyl)-vinyl ether 936-69-6 C8H6Cl2O 189.041 —— 2,4-dichlorophenoxymethyl chloride 13543-09-4 C7H5Cl3O 211.475 —— 2,4-dichloro-α-iodoanisole 106262-00-4 C7H5Cl2IO 302.927 2,4-二氯-1-苯氧基-苯 2,4-dichloro-diphenyl ether 51892-26-3 C12H8Cl2O 239.101 —— 2,4,4'-Trichlorobiphenyl ether 59039-21-3 C12H7Cl3O 273.546 4,6-二氯-2-氨基苯酚 2,4-dichloro-6-aminophenol 527-62-8 C6H5Cl2NO 178.018 2,4-二氯-6-碘苯酚 2,4-dichloro-6-iodophenol 2040-83-7 C6H3Cl2IO 288.9 2,6-二氯-4-溴酚 2-bromo-4,6-dichlorophenol 4524-77-0 C6H3BrCl2O 241.899 1,1'-氧基二(2,4-二氯苯) 2,2',4,4'-tetrachlorodiphenylether 28076-73-5 C12H6Cl4O 307.991 —— (2,4-dichloro-phenyl)-propyl ether 6851-39-4 C9H10Cl2O 205.084 —— dichloro-(2,4-dichloro-phenoxy)-phosphine 69477-35-6 C6H3Cl4OP 263.875 2 - (2,4-二氯苯氧基)乙醇 2-(2,4-dichlorophenoxy)ethanol 120-67-2 C8H8Cl2O2 207.056 —— 2,4-(dichloroisopropoxy)benzene 6851-40-7 C9H10Cl2O 205.084 —— 2-(2,4-dichlorophenoxy)ethyl chloride 64010-13-5 C8H7Cl3O 225.502 1-(2-溴甲氧基)-2,4-二氯苯 1-(2-bromoethoxy)-2,4-dichlorobenzene 6954-77-4 C8H7BrCl2O 269.953 2,4-二氯苯基丙炔基醚 2,4-dichloro-1-(prop-2-yn-1-yloxy)benzene 17061-90-4 C9H6Cl2O 201.052 —— 1-(allyloxy)-2,4-dichlorobenzene 5441-16-7 C9H8Cl2O 203.068 2,4-二氯苯氧基乙腈 2-(2,4-dichlorophenoxy)acetonitrile 3956-63-6 C8H5Cl2NO 202.04 2,4-二氯-1-(二氟甲氧基)苯 2,4-di-chloro-1-(difluoromethoxy)benzene 772-23-6 C7H4Cl2F2O 213.011 2-(2,4-二氯-苯氧基)-乙胺 2-(2,4-dichlorophenoxy)ethylamine 1199-28-6 C8H9Cl2NO 206.072 —— (2,4-dichlorophenoxy)acetaldehyde 17944-27-3 C8H6Cl2O2 205.04 —— bis(2,4-dichlorophenoxy)methane 35412-53-4 C13H8Cl4O2 338.017 (2,4-二氯苯氧基)丙醇 3-(2,4-dichloro-phenoxy)-propan-1-ol 56927-95-8 C9H10Cl2O2 221.083 1,2-双(2,4二氯苯氧基)乙烷 1,2-Bis-(2,4-dichlor-phenoxy)-ethan 6339-70-4 C14H10Cl4O2 352.044 —— (2,4-dichloro-phenyl)-isobutyl ether 6851-42-9 C10H12Cl2O 219.111 3-氯苯酚 3-Chlorophenol 108-43-0 C6H5ClO 128.558 —— butyl 2,4-dichlorophenolate 6851-41-8 C10H12Cl2O 219.111 —— 1-(but-3-yn-1-yloxy)-2,4-dichlorobenzene 1339089-58-5 C10H8Cl2O 215.079 —— 3-(2,4-dichlorophenoxy)propan-1-amine 50911-63-2 C9H11Cl2NO 220.098 1-(3-溴丙氧基)-2,4-二氯苯 1-(3-bromopropoxy)-2,4-dichlorobenzene 6954-78-5 C9H9BrCl2O 283.98 —— (2,4-Dichlor-phenoxy)-trimethyl-silan 17878-30-7 C9H12Cl2OSi 235.185 1,3-二氯-4-三氟甲氧基苯 1,3-dichloro-4-(trifluoromethoxy)benzene 451-85-4 C7H3Cl2F3O 231.001 —— 3-(2,4-dichloro-phenoxy)-propionitrile 33695-72-6 C9H7Cl2NO 216.067 —— 1-ethoxymethoxy-2,4-dichloro-benzene 829-59-4 C9H10Cl2O2 221.083 —— 2,4-dichlorophenyl pentyl ether 63076-61-9 C11H14Cl2O 233.138 —— (2,4-dichloro-phenyl)-isopentyl ether 6851-49-6 C11H14Cl2O 233.138 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:参考文献:名称:氯醛与卤代苯和 2,4-二氯苯的反应:卤代苯和 α-甲氧基苯乙酸的合成摘要:综述了氯醛与芳香族化合物生成芳基三氯甲基甲醇的 Friedel-Crafts 反应。氯化铝与氯醛的摩尔比很关键,从 0.15 到...DOI:10.1139/v66-078
-
作为产物:参考文献:名称:Electroreduction of Organic Compounds, 34 [1]. Cathodic Dehalogenation of Chloroarenes with Electron-Donating Substituents摘要:氯代芳烃的电化学还原与供电取代基(如氯甲苯、-苯甲醚和-酚)进行研究。在铅或碳阴极下,在各种溶剂支持电解质的电位静态和电流静态条件下进行制备电解。在适当条件下,可能对具有两个或更多氯取代基的化合物进行部分和主要区域选择性的去氯化。尤其是在对位,替换一个单独的氯取代基是困难的。因此,高度有毒和持久的寡氯衍生物可以转化为氯化程度较低的问题较少的化合物。通过电还原,也可以显着降低受氯化酚和Nitrofen®污染的土壤提取物等实际材料的氯含量。DOI:10.1515/znb-2003-1206
-
作为试剂:参考文献:名称:具有抗炎活性的取代的(2-苯氧基苯基)乙酸。1。摘要:描述了一系列取代的(2-苯氧基苯基)乙酸的合成和抗炎活性。在佐剂关节炎试验中的初步筛选显示苯氧基环中的卤素取代大大增强了活性。用最小致溃疡剂量(MUD)衡量的致溃疡潜力在几乎所有测试的酸中均很低。[2-(2,4-二氯苯氧基)苯基]乙酸具有最有利的效价和低毒性,包括致溃疡性,该化合物现已用于治疗。DOI:10.1021/jm00364a004
文献信息
-
[EN] NOVEL THYROMIMETICS<br/>[FR] NOUVEAUX THYROMIMÉTIQUES申请人:AUTOBAHN THERAPEUTICS INC公开号:WO2021108549A1公开(公告)日:2021-06-03Compounds are provided having the structure of Formula (I) or a pharmaceutically acceptable isomer, racemate, hydrate, solvate, isotope, or salt thereof, wherein R1, R2, X1, X2, Y1, and Y2 are as defined herein. Such compounds function as thyromimetics and have utility for treating diseases such as neurodegenerative disorders and fibrotic diseases. Pharmaceutical compositions containing such compounds are also provided, as are methods of their use and preparation.提供具有Formula (I)结构或其药学上可接受的异构体、拉克酸盐、水合物、溶剂化合物、同位素或盐的化合物,其中R1、R2、X1、X2、Y1和Y2如本文所定义。这些化合物作为甲状腺类似物发挥作用,并可用于治疗神经退行性疾病和纤维化疾病等疾病。还提供含有这些化合物的药物组合物,以及它们的使用和制备方法。
-
A Novel and Versatile Silicon-Derived Linkage Agent, 4-[1-Hydroxy-2-(trimethylsilyl)ethyl]benzoic Acid, Compatible with the Fmoc/t-Bu Strategy for Solid Phase Synthesis of C-Terminal Peptide Acids作者:Hann-Guang Chao、Michael S. Bernatowicz、Paul D. Reiss、Clifford E. Klimas、Gary R. MatsuedaDOI:10.1021/ja00084a016日期:1994.3solid-phase peptide synthesis. With the development of a versatile silicon-based linkage agent, «SAC», 4-[1-hydroxy-2-(trimethylsilyl)ethyl] benzoic acid (1), for the production of peptide C-terminal acids, these problems have been successfully solved. Demonstration of the advantages of using SAC linker was provided by synthesizing a C-terminal tryptophan-containing dodecapeptide and a C-terminal proline-containing通过树脂衍生的碳正离子进行色氨酸烷基化和二酮哌嗪的形成是在基于 Fmoc/t-Bu 的固相肽合成中观察到的两个主要的产率降低副反应。随着多功能硅基连接剂 «SAC»、4-[1-羟基-2-(三甲基甲硅烷基)乙基]苯甲酸 (1) 的开发,用于生产肽 C 端酸,这些问题已得到解决成功解决。通过合成含有 C 端色氨酸的十二肽和含有 C 端脯氨酸的十一肽,证明了使用 SAC 接头的优势。纯肽以 30-40% 的产率分离,与使用传统 HMPA 接头的合成相比,这是一个显着的改进。SAC 接头分两步制备,总产率为 70%。Fmoc-氨基酸-SAC 接头衍生物与氨基官能化固体支持物的连接最好使用所实施的 2,4-二氯苯基酯衍生物进行。测试了 Tha 氨基酸-SAC 键并发现在典型的肽合成条件下是稳定的。SAC 接头的另一个特点是能够使用氟离子或 1% TFA/CH 2 Cl 2 溶液生成受保护的肽片段。通过氟解方法或用稀酸处理制备几种
-
1,1,2,2-Tetrahydroperoxy-1,2-Diphenylethane: An efficient and high oxygen content oxidant in various oxidative reactions作者:Kaveh Khosravi、Shirin NaserifarDOI:10.1016/j.tet.2018.09.041日期:2018.11Several oxidative approaches namely thiocyanation of aromatic compounds, epoxidation of alkenes, amidation of aromatic aldehydes, epoxidation of α, β-unsaturated ketones, oxidation of sulfides to sulfoxides and sulfones, bayer-villeger reaction, bromination and iodation of aniline and phenol derivatives oxidative esterification, oxidation of pyridines and oxidation of secondary, allylic and benzyllic
-
Syntheses of Benzo[d]Thiazol-2(3H)-One Derivatives and Their Antidepressant and Anticonvulsant Effects作者:Qinghao Jin、Zhiyang Fu、Liping Guan、Haiying JiangDOI:10.3390/md17070430日期:——Thirty-four new benzo[d]thiazol derivatives 2a-2i, 3a-3r, and 4a-4g were synthesized and investigated for their potential antidepressant and anticonvulsant effects. In a forced swimming test, 2c and 2d showed the highest antidepressant and anticonvulsant effects. 2c and 2d displayed a higher percentage decrease in immobility duration (89.96% and 89.62%, respectively) than that of fluoxetine (83.62%)
-
Reactivity of new precursors of quinone methides作者:Bernard Loubinoux、Joseph Miazimbakana、Philippe GerardinDOI:10.1016/s0040-4039(00)99619-9日期:1989.1The azidomethylene protecting group allows the synthesis of unstable phenolic compounds which are used as quinone methide precursors in the alkylations of alcohols, phenols, azide, thiophenol, amines, enols and enolates.
表征谱图
-
氢谱1HNMR
-
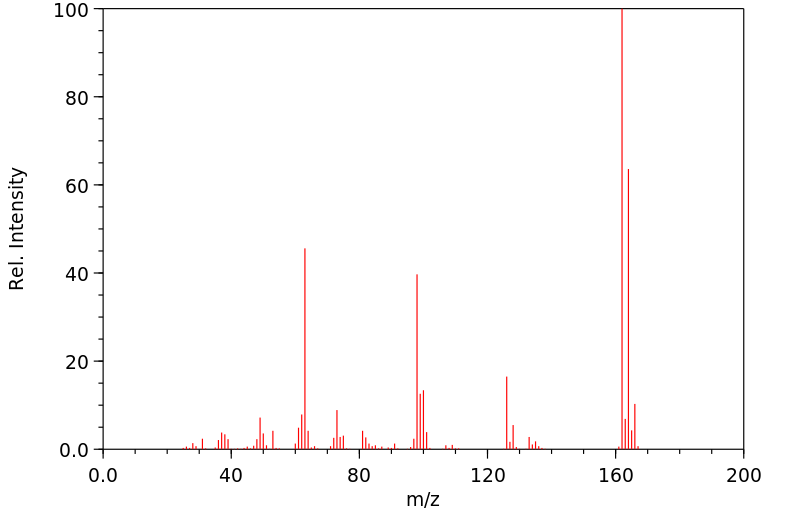
质谱MS
-
碳谱13CNMR
-
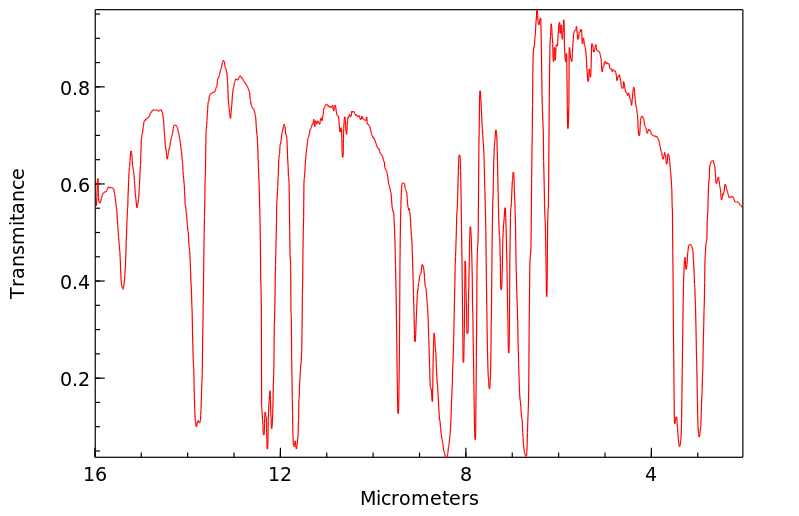
红外IR
-
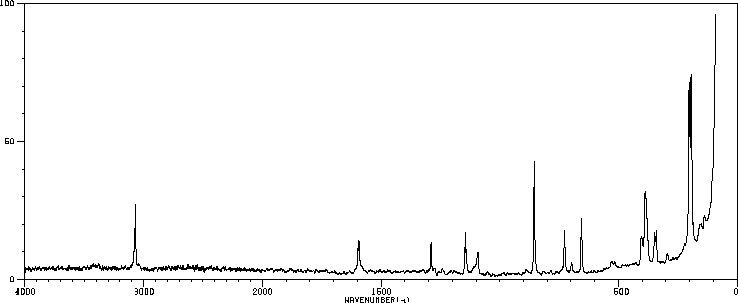
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫