2,6-二碘对苯醌 | 20389-01-9
中文名称
2,6-二碘对苯醌
中文别名
2,6-二碘-邻-苯醌
英文名称
2,6-diiodo-p-benzoquinone
英文别名
2,6-Diiodoquinone;2,6-diiodo-[1,4]benzoquinone;2,6-Dijod-[1,4]benzochinon;2.6-Dijod-chinon;2,6-diiodocyclohexa-2,5-diene-1,4-dione
CAS
20389-01-9
化学式
C6H2I2O2
mdl
MFCD00013786
分子量
359.89
InChiKey
KANJSWOVFIVFDI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177.5°C
-
沸点:312.8±42.0 °C(Predicted)
-
密度:2.6051 (estimate)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R20/22,R36/37/38
-
海关编码:2914700090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Wunderer,H., Chemische Berichte, 1972, vol. 105, p. 3479 - 3490摘要:DOI:
-
作为产物:描述:参考文献:名称:An Optical Method for the Study of Reversible Organic Oxidation—Reduction Systems. III. Preparation and Use of a New Optically Active Standard摘要:DOI:10.1021/ja01318a039
文献信息
-
Thallium in organic synthesis—XVI作者:A. McKillop、B.P. Swann、E.C. TaylorDOI:10.1016/s0040-4020(01)93043-1日期:1970.1Treatment of 2,6-disubstituted-4-t-butylphenols with thallium(III) trifluoroacetate in either trifluoroacetic acid or carbon tetrachloride as solvent results in loss of the 4-t-butyl substituent as isobutene and formation in high yield of the corresponding 2,6-disubstituted p-quinones. Possible mechanisms for this novel reaction, which probably proceeds via the intermediacy of a hydroquinone or hydroquinone
-
Halogen‐Mediated Membrane Transport: An Efficient Strategy for the Enhancement of Cellular Uptake of Synthetic Molecules作者:Harinarayana Ungati、Vijayakumar Govindaraj、Chithra R. Nair、Govindasamy MugeshDOI:10.1002/chem.201806122日期:——biological molecules require specific membrane transporters and channel proteins that control the traffic of these molecules into and out of the cell. This work reports that the introduction of halogen atoms into a series of fluorescent molecules remarkably enhances their cellular uptake, and that their transport can be increased to more than 95 % by introducing two iodine atoms at appropriate positions. The哺乳动物细胞对荧光探针和治疗剂的吸收不良是生物学应用中的一个主要问题,涉及从荧光成像到活细胞内药物传递的各种领域。尽管气态分子(例如氧气和二氧化碳),疏水性物质(例如苯)以及小的极性但不带电荷的分子(例如水和乙醇)可以通过简单的被动扩散穿过细胞质膜,但许多合成分子和生物分子都需要特定的膜转运蛋白和控制这些分子进出细胞的通道蛋白。这项工作报告说,将卤素原子引入一系列荧光分子中可以显着增强其细胞摄取,并且通过在适当位置引入两个碘原子,可以将其转运提高到95%以上。当分子中存在碘原子时,荧光团的性质在细胞摄取中并不起主要作用,因为带有萘二甲酰亚胺,香豆素,BODIPY和pyr部分的化合物显示出相似的摄取。有趣的是,引入带有两个羟乙基硫基的马来酰亚胺基荧光团使分子能够穿过质膜和核膜,碘原子的存在进一步增强了跨这两个膜的传输。总的来说,这项研究提供了增强哺乳动物细胞对有机分子吸收的一般策略。引入带有
-
Cross-Linking and Sequence Specific Alkylation of DNA by Aziridinyl Quinones. 2. Structure Requirements for Sequence Selectivity作者:Rob H. J. Hargreaves、Stephen P. Mayalarp、John Butler、Simon R. McAdam、C. Caroline O'Hare、John A. HartleyDOI:10.1021/jm960492j日期:1997.1.1The cytotoxicities and DNA sequence selectivity for guanine-N7 alkylation of 22 mono- and disubstituted 2,5-diaziridinyl-1,4-benzoquinones have been investigated. Several quinones produced patterns of alkylation following reduction with a selectivity for 5'-TGC-3' sequences. This sequence selectivity appeared to be dependent only on the presence of a hydrogen in position-6 of the quinone. A computer
-
3,6-二(丙-2-亚基)环己-1,4-二烯衍生物及其制备方法、应用和器件
-
Generation of skeletal diversity within a combinatorial library申请人:——公开号:US20040214232A1公开(公告)日:2004-10-28The present invention provides a method of synthesizing a library of chemical compounds with skeletal diversity. Two approaches are used to create skeletal diversity within a library of chemical compounds: (1) the “branching pathways” (or reagent-based) approach; and (2) the “folding pathways” (or substrate-based) approach. Upon exposure to certain reaction conditions the members of the library undergo unique transformations into a diverse collection of molecular skeletons, which can be functionalized and derivatized further to generate a large collection of unique, natural product-like compounds. A furan-based library synthesized using the folding pathways approach is provided, and a polycyclic library created using the braching pathways approach is also provided. The invention also provides materials, reagents, intermediates, and kits useful in the practice of the inventive method as well as method for screening the inventive compounds.
表征谱图
-
氢谱1HNMR
-
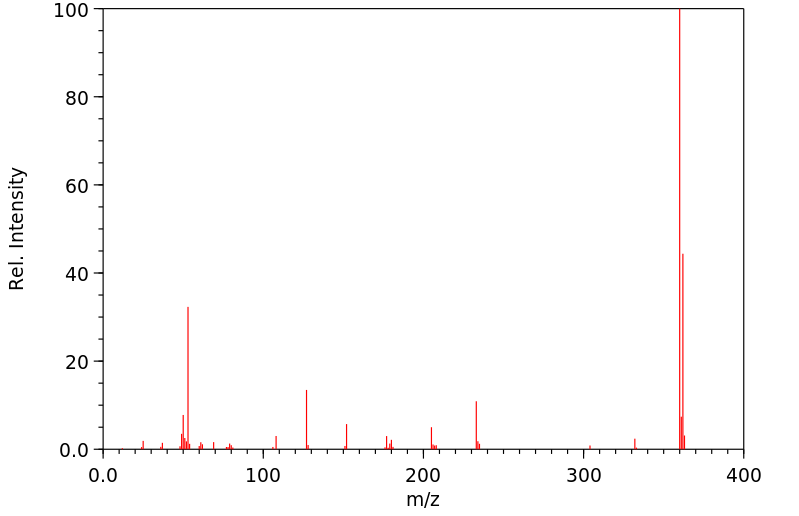
质谱MS
-
碳谱13CNMR
-
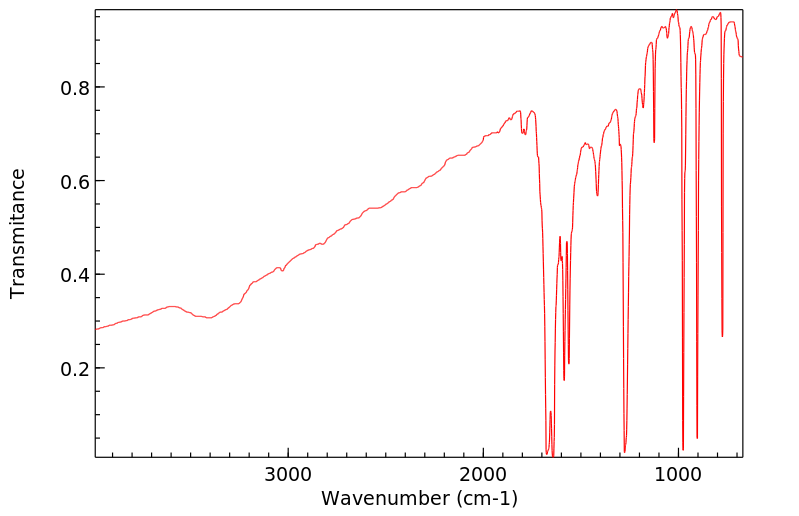
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷