2-呋喃基GLY氧基O腈 | 6047-91-2
中文名称
2-呋喃基GLY氧基O腈
中文别名
2-呋喃基甲酰腈
英文名称
2-furoyl cyanide
英文别名
furan-2-carbonyl cyanide
CAS
6047-91-2
化学式
C6H3NO2
mdl
MFCD00042670
分子量
121.095
InChiKey
BITJKGFKDMCINV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:233-236 °C
-
沸点:85-87°C 12mm
-
密度:1.246
-
闪点:60℃
-
溶解度:可溶于DMSO(少许)、乙酸乙酯(少许)
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:54
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R20/21/22
-
海关编码:2932190090
SDS
| Name: | Alpha-Oxo-2-Furanacetonitrile Material Safety Data Sheet |
| Synonym: | 2-Furoyl cyanide |
| CAS: | 6047-91-2 |
Synonym: 2-Furoyl cyanide
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6047-91-2 | alpha-Oxo-2-furanacetonitrile | 227-944-8 |
Risk Phrases: 20/21/22
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Harmful by inhalation, in contact with skin and if swallowed. Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation.
Chronic:
Not available.
SECTION 4 - FIRST AID MEASURES
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
SECTION 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 6047-91-2: Personal Protective Equipment
Eyes:
Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 85-87 deg C @ 12 mmHg
Freezing/Melting Point: 19 - 22 deg C
Autoignition Temperature: Not available.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.180
Molecular Formula: C6H3NO2
Molecular Weight: 121.1
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 6047-91-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
alpha-Oxo-2-furanacetonitrile - Not listed by ACGIH, IARC, or NTP.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class: 6.1
UN Number:
Packing group: III
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and gloves. WGK (Water Danger/Protection) CAS# 6047-91-2: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 6047-91-2 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 6047-91-2 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 3/20/2003 Revision #0 Date: Original. The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:2-呋喃基GLY氧基O腈 在 四(三苯基膦)钯 作用下, 反应 12.0h, 以98%的产率得到2-氰基呋喃参考文献:名称:Palladium-catalyzed decarbonylation of acyl cyanides摘要:DOI:10.1021/jo00356a029
-
作为产物:描述:4,4,4-三氟-1-(2-呋喃基)-1,3-丁二酮 在 柠檬酸 、 sodium nitrite 、 乙酸酐 作用下, 以 水 、 氯仿 为溶剂, 反应 2.0h, 以78%的产率得到2-呋喃基GLY氧基O腈参考文献:名称:4,4,4-三氟-3,3-二羟基-2-(羟基亚氨基)丁-1-酮的三氟乙酰化作为酰基氰化物的简便合成策略摘要:氟化的1,3-二羰基化合物与NaNO 2在酸性条件下的反应重新研究表明,形成了相应的1,1,1-三氟-3-羟基亚氨基-丁酮-2,4-二酮,其主要以水合物形式分离。描述了通过酸催化的获得的1,3-二羰基化合物的2-羟基亚氨基衍生物的乙氧基-,烷基-,(杂)芳基取代的羰基氰化物的新颖合成。DOI:10.1016/j.jfluchem.2016.04.009
文献信息
-
Proline-catalyzed aldol reactions of acyl cyanides with acetone: an efficient and convenient synthesis of 1,3-diketones作者:Zongxuan Shen、Bin Li、Lu Wang、Yawen ZhangDOI:10.1016/j.tetlet.2005.10.036日期:2005.12The aldol-type addition of acetone towards (un)substituted benzoyl, heteroarylcarbonyl or α,β-unsaturated acyl cyanides was efficiently catalyzed by l-proline (30 mol %) to give 2-hydroxy-4-oxo-2-substituted pentanenitriles. Upon the treatment with sodium hydroxide, the adducts transformed to 1,3-diketones in good-to-excellent yield, furnishing an efficient and convenient method for the regioselective
-
Cp*RuCl-Catalyzed [2 + 2 + 2] Cycloadditions of α,ω-Diynes with Electron-Deficient Carbon−Heteroatom Multiple Bonds Leading to Heterocycles作者:Yoshihiko Yamamoto、Keisuke Kinpara、Tomoaki Saigoku、Hideyuki Takagishi、Satoshi Okuda、Hisao Nishiyama、Kenji ItohDOI:10.1021/ja045694g日期:2005.1.1In the presence of a catalytic amount of Cp*RuCl(cod), 1,6-diynes were allowed to react chemo- and regioselectively with electron-deficient nitriles and heterocumulenes at 60−90 °C to afford heterocyclic compounds. The mechanism of the ruthenium-catalyzed regioselective formations of bicyclic pyridines and pyridones were analyzed on the basis of density functional calculations. Cyclocotrimerizations
-
Reaction of Aromatic Acyl Chlorides with Potassium or Sodium Cyanide Impregnated onto Amberlite XAD Resins. Efficient Synthesis of Aromatic Acyl Cyanides作者:Kazuaki SukataDOI:10.1246/bcsj.60.1085日期:1987.3The effects of alkali metal cyanide impregnated on Amberlite XAD resins (KCN/XAD, NaCN/XAD) have been examined using the cyanation of benzoyl chloride. In benzene, benzoyl cyanide was obtained in a very high yield with high selectivity under mild conditions. It is proposed that the reaction occurs on the surface of the resin. On the basis of the result obtained in the absence of any solvent, the reactivity
-
AgI-PEG400-KI Catalyzed Environmentally Benign Synthesis of Aroyl Cyanides Using Potassium Hexacyanoferrate(II) as the Cyanating Agent作者:Zheng Li、Shengyi Shi、Jingya YangDOI:10.1055/s-2006-950407日期:2006.9A practical cyanation of aroyl chlorides with 0.2 equivalent of non-toxic cyanide source, K 4 [Fe(CN) 6 ], 3 mol% AgI, 4 mol% PEG-400, and 3 mol% KI as the catalyst system is described. The reactions were performed in DMF at room temperature and provided the corresponding aroyl cyanides in 64-89% yield, typically in less than ten hours.
-
Indium-mediated reductive coupling of acyl cyanides: a convenient synthesis of 1,2-diketones作者:Heung Soo Baek、Sung Jae Lee、Byung Woo Yoo、Jae Jung Ko、Sung Hoon Kim*、Joong Hyup KimDOI:10.1016/s0040-4039(00)01411-8日期:2000.10The indium-mediated reductive coupling of acyl cyanides afforded the corresponding 1,2-diketones in moderate to good yields under neutral and mild conditions.
表征谱图
-
氢谱1HNMR
-
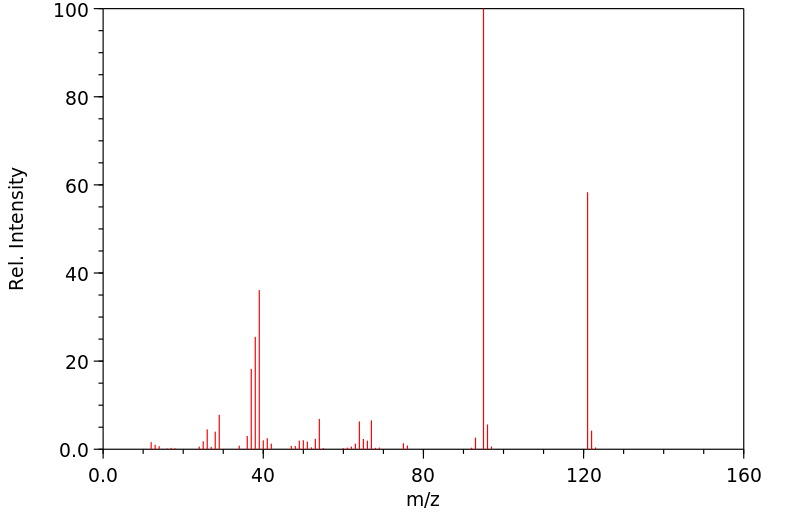
质谱MS
-
碳谱13CNMR
-
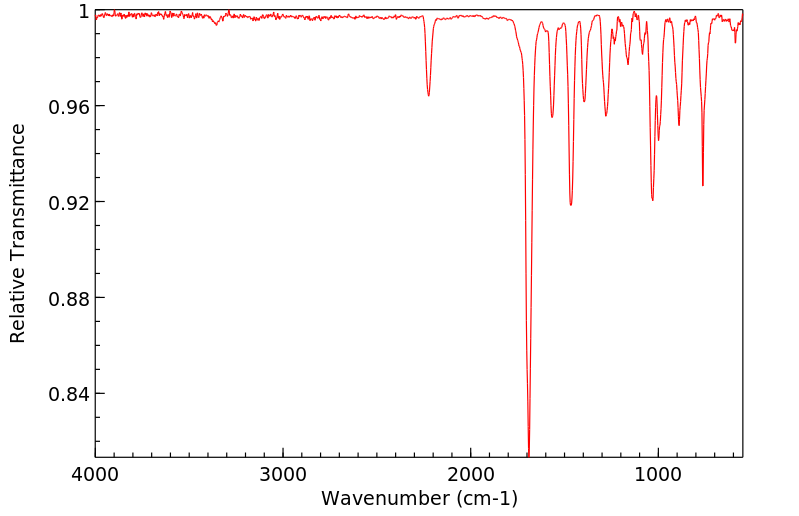
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷