球松素 | 480-37-5
中文名称
球松素
中文别名
5-羟基-7-甲氧基-2-苯基色满-4-酮;5-羟基-7-甲氧基黄酮(吡P醇);乔松酮;5-羟基-7-甲氧基黄烷酮
英文名称
pinostrobin
英文别名
5-hydroxy-7-methoxyflavanone;pinocembrin-7-methyl ether;(2S)-5-hydroxy-7-methoxyflavanone;S-(-)-pinostrobin;(2S)-pinostrobin;(2S)-5-hydroxy-7-methoxy-2-phenyl-2,3-dihydrochromen-4-one;(S)-5-hydroxy-7-methoxyflavanone;Dihydrotectochrysin
CAS
480-37-5
化学式
C16H14O4
mdl
——
分子量
270.285
InChiKey
ORJDDOBAOGKRJV-AWEZNQCLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100°C
-
沸点:494.9±45.0 °C(Predicted)
-
密度:1.284±0.06 g/cm3(Predicted)
-
溶解度:DMF:20mg/mL; DMF:PBS (pH 7.2)(1:3):0.25 mg/ml;二甲基亚砜:12mg/mL
-
LogP:3.591 (est)
-
碰撞截面:164.24 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:20
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:55.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2932999099
SDS
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— dimethylpinocembrin —— C17H16O4 284.312 —— (R)-3-hydroxy-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-phenylpropan-1-one 862254-91-9 C17H18O5 302.327 —— pinostrobin 5-β-D-glucoside 115799-14-9 C22H24O9 432.427 —— (R)-1-[2,4-dimethoxy-6-(methoxymethoxy)phenyl]-3-phenyl-3-(tert-butyldimethylsilyl)oxy-propan-1-one 351458-87-2 C25H36O6Si 460.643
反应信息
-
作为反应物:参考文献:名称:Dibromopinocembrin 和 Dibromopinostrobin 是潜在的抗登革热药物,具有轻微的动物毒性摘要:登革热感染是全球 20 亿面临风险的最有害的公共卫生问题之一。严重者的血浆渗漏、出血和休克是由于继发性异型感染引起的免疫紊乱所致。黄烷酮通常存在于药用植物中,以前因其直接抗病毒作用和抑制登革热免疫发病机制中的促炎细胞因子而显示出作为抗登革热抑制剂的潜力。在这里,我们通过卤化对黄烷酮、松香素和松果酯进行了化学修饰,并将它们表征为潜在的登革热 2 抑制剂,并在人源细胞和体内动物模型中进行了毒性测试。Dibromopinocembrin 和 dibromopinostrobin 在 EC50 为 2.0640 ± 0.7537 和 5.8567 ± 0.5074 µM 时抑制登革热血清型 2,在 CC50 为 67.2082 ± 0.9731 和 > 分别为 100 µM。两种化合物对成年 C57BL/6 小鼠的毒性也很小,通过静脉给药后第一天、第三天和第八天的 ALT 和 Cr 水平进行评估。计算研究表明潜在目标可能是DOI:10.3390/molecules25184154
-
作为产物:参考文献:名称:通过铑催化的不对称1,4加成反应高效合成对黄酮类化合物,包括松果糖醇摘要:有效的合成生物活性手性黄烷酮(1)是通过Rh催化的芳基硼酸到色酮的不对称1,4-加成反应而实现的。在室温下,在0.5%Rh催化剂和贫电子手性二膦MeO-F 12 -BIPHEP的存在下,甲苯中的反应平稳进行。在该反应中,经常发生向(S)-1的1,2-加成反应生成副产物(2 S,4 R)-2,4-二芳基-4-苯并二氢呋喃,可通过改变反应溶剂来减少CH 2 Cl 2使Rh催化剂失活(需要3%)。DOI:10.1021/ol2004148
文献信息
-
Approaches to 2-substituted chroman-4-ones: synthesis of (−)-pinostrobin作者:Kevin J HodgettsDOI:10.1016/s0040-4039(01)00567-6日期:2001.5Two approaches to optically active 2-substituted chroman-4-ones are described. The first utilized the oxidation of a preformed chroman ring and the second an intramolecular Mitsunobu cyclization. The methodology was applied to the synthesis of the biologically active natural product (−)-pinostrobin (18).
-
Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and β-Cyclodextrins作者:Jintawee Kicuntod、Kanyani Sangpheak、Monika Mueller、Peter Wolschann、Helmut Viernstein、Saeko Yanaka、Koichi Kato、Warinthorn Chavasiri、Piamsook Pongsawasdi、Nawee Kungwan、Thanyada RungrotmongkolDOI:10.3390/scipharm86010005日期:——Pinostrobin (PNS) belongs to the flavanone subclass of flavonoids which shows several biological activities such as anti-inflammatory, anti-cancerogenic, anti-viral and anti-oxidative effects. Similar to other flavonoids, PNS has a quite low water solubility. The purpose of this work is to improve the solubility and the biological activities of PNS by forming inclusion complexes with β-cyclodextrin (βCD) and its derivatives, heptakis-(2,6-di-O-methyl)-β-cyclodextrin (2,6-DMβCD) and (2-hydroxypropyl)-β-cyclodextrin (HPβCD). The AL-type diagram of the phase solubility studies of PNS exhibited the formed inclusion complexes with the 1:1 molar ratio. Inclusion complexes were prepared by the freeze-drying method and were characterized by differential scanning calorimetry (DSC). Two-dimensional nuclear magnetic resonance (2D-NMR) and steered molecular dynamics (SMD) simulation revealed two different binding modes of PNS, i.e., its phenyl- (P-PNS) and chromone- (C-PNS) rings preferably inserted into the cavity of βCD derivatives whilst only one orientation of PNS, where the C-PNS ring is inside the cavity, was detected in the case of the parental βCD. All PNS/βCDs complexes had a higher dissolution rate than free PNS. Both PNS and its complexes significantly exerted a lowering effect on the IL-6 secretion in LPS-stimulated macrophages and showed a moderate cytotoxic effect against MCF-7 and HeLa cancer cell lines in vitro.Pinostrobin(PNS)属于黄酮类化合物中的黄烷酮亚类,具有多种生物活性,如抗炎、抗癌、抗病毒和抗氧化作用。与其他黄酮类化合物类似,PNS的水溶性极低。本研究的目的是通过与β-环糊精(βCD)及其衍生物、七聚(2,6-二-O-甲基)-β-环糊精(2,6-DMβCD)和(2-羟丙基)-β-环糊精(HPβCD)形成包合复合物,提高PNS的溶解度和生物活性。PNS的相溶性研究的AL型图显示了以1:1摩尔比形成的包合复合物。包合复合物通过冷冻干燥法制备,并通过差示扫描量热法(DSC)进行表征。二维核磁共振(2D-NMR)和受控分子动力学(SMD)模拟揭示了PNS的两种不同结合模式,即苯基(P-PNS)和色酮(C-PNS)环优先插入βCD衍生物的空腔中,而在母体βCD的情况下,仅检测到一种PNS取向,即C-PNS环位于空腔内。所有PNS/βCD复合物的溶解速率均高于游离PNS。PNS及其复合物均对LPS刺激的巨噬细胞中的IL-6分泌具有
-
Benzopyran phenol derivates for use as antibacterial, antiviral or immunostimulating agents申请人:Fockerman, Jasmine公开号:EP0707851A2公开(公告)日:1996-04-24The invention concerns the use of compounds with the general formula I where R is the same or different and represents H, OH or for preparing a composition for use as an antibacterial, antiviral or immunostimulating agent or wound healing factor. A process for preparing a mixture containing these substances is disclosed, whereby a propolis containing product such as an organic solvent, preferably an alcoholic solution con- taining propolis, is added to water or a water solution containing 0,1-17 weight % of NaCl with a temperature of 30-95oC, and the mixture is kept at 30-95oC for 10-100 hours, whereafter the solution is freed from the bottom sediment.
-
Kava derived therapeutic compounds and methods of use thereof申请人:KUALITY HERBCEUTICS LLC公开号:US10624943B2公开(公告)日:2020-04-21Certain embodiments of the invention provide a composition comprising at least two compounds selected from the group consisting of dihydromethysticin, methysticin, dihydrokavain, kavain, desmethoxyyangonin and 11-methoxyyangonin, wherein the composition is substantially free of flavokawain B. Certain embodiments of the invention also provide a method for treating or preventing cancer in a mammal (e.g., a human) in need of such treatment comprising, administering to the mammal a carrier and a compound selected from the group consisting of dihydromethysticin, methysticin, dihydrokavain, kavain, desmethoxyyangonin and 11-methoxyyangonin, wherein the compound is substantially free of other kava extract components.
-
Erdtman, Svensk Kemisk Tidskrift, 1944, vol. 56, p. 95,99作者:ErdtmanDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
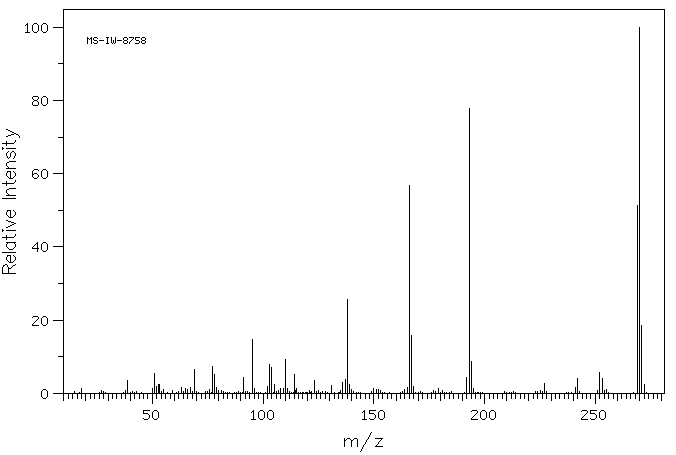
质谱MS
-
碳谱13CNMR
-
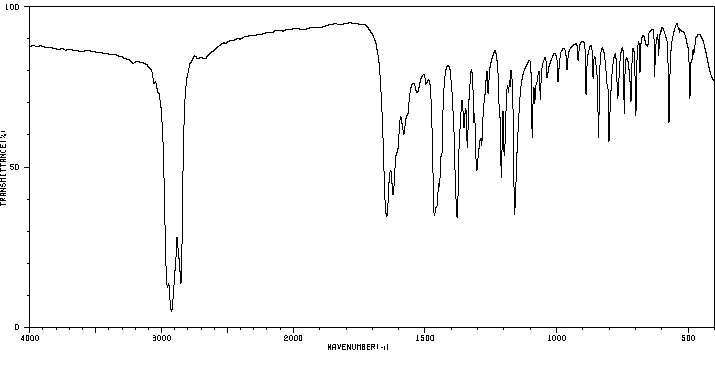
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷