2-羟基异丁酰胺 | 13027-88-8
中文名称
2-羟基异丁酰胺
中文别名
2-甲基乳酰胺;2-羟基-2-甲基丙酰胺;2-羟基异丁酸酰胺
英文名称
2-hydroxyisobutyramide
英文别名
alpha-hydroxyisobutyramide;α-hydroxy-isobutyramide;2-hydroxy-2-methylpropanamide;α-Hydroxy-isobutyramid;2-hydroxy-2-methylpropionamide;α-Hydroxy-isobuttersaeure-amid;2-Hydroxy-2-methyl-propionsaeureamid;2-methyl-2-hydroxypropionamide;Propanamide, 2-hydroxy-2-methyl-
CAS
13027-88-8
化学式
C4H9NO2
mdl
——
分子量
103.121
InChiKey
DRYMMXUBDRJPDS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93 °C
-
沸点:260 °C
-
密度:1.109±0.06 g/cm3(Predicted)
-
溶解度:少量溶于甲醇和水
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:63.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
WGK Germany:1
-
RTECS号:RP4489165
-
海关编码:2924199090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温且干燥
SDS
2-羟基异丁酰胺 修改号码:5
模块 1. 化学品
产品名称: 2-Hydroxyisobutyramide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-羟基异丁酰胺
百分比: >98.0%(N)
CAS编码: 13027-88-8
俗名: 2-Hydroxy-2-methylpropionamide
2-羟基异丁酰胺 修改号码:5
模块 3. 成分/组成信息
分子式: C4H9NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
2-羟基异丁酰胺 修改号码:5
模块 9. 理化特性
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
93°C
沸点/沸程 260 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
2-羟基异丁酰胺 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Hydroxyisobutyramide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-羟基异丁酰胺
百分比: >98.0%(N)
CAS编码: 13027-88-8
俗名: 2-Hydroxy-2-methylpropionamide
2-羟基异丁酰胺 修改号码:5
模块 3. 成分/组成信息
分子式: C4H9NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
2-羟基异丁酰胺 修改号码:5
模块 9. 理化特性
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
93°C
沸点/沸程 260 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
2-羟基异丁酰胺 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-methoxy-2-methylpropanamide 82473-56-1 C5H11NO2 117.148
反应信息
-
作为反应物:参考文献:名称:DEUTERATED BENZIMIDAZOLE COMPOUND AND MEDICAL USE THEREOF摘要:该发明涉及一种用于治疗或预防涉及Na通道的疾病的药物,例如神经痛、伤害性疼痛、炎症性疼痛、小纤维神经病、红细胞增多症、阵发性极端疼痛障碍、排尿困难或多发性硬化等,包括式(I)的化合物,其中R1a、R1b、R1c和R1d为氢、卤素、氰基、C1-4烷基、C1-4烷氧基等,但至少其中一个为上述的C6-10芳基、C6-10芳氧基等,R2和R3为氢、C1-6烷基、C3-10环烷基等,R4为氢、C1-6烷基、C3-7环烷基等,m为0、1或2,L为CR7R8,R7和R8为氢、羟基、C1-4烷基、C1-4烷氧基等,或其药学上可接受的盐。公开号:US20200017450A1
-
作为产物:描述:参考文献:名称:氰醇催化转移水合为 α-羟基酰胺摘要:我们报告了通过使用羧酰胺作为水供体,钯 (II) 催化的氰醇转移水合为 α-羟基酰胺。该方法能够在温和条件下(50°C,10 分钟)选择性地水合各种醛和酮衍生的氰醇,分别提供 α-单-和 α,α-二取代-α-羟基酰胺。非诺贝特(一种带有二苯甲酮部分的药物)直接转化为功能化的 α,α-二芳基-α-羟基酰胺是通过氢氰化-转移水合序列实现的。初步动力学研究和位点特异性 18O 标记的 α-羟基酰胺的合成证明了羰基氧从羧酰胺试剂转移到 α-羟基酰胺产物中。DOI:10.1021/jacs.8b12877
文献信息
-
Hydration of Cyanohydrins by Highly Active Cationic Pt Catalysts: Mechanism and Scope作者:Chengcheng Li、Xiao-Yong Chang、Luqiong Huo、Haibo Tan、Xiangyou Xing、Chen XuDOI:10.1021/acscatal.1c02254日期:2021.7.16Cyanohydrins (α-hydroxy nitriles) are a special type of nitriles that readily decompose into hydrogen cyanide (HCN) and the corresponding carbonyl compounds. Hydration of cyanohydrins that are readily available through cyanation of aldehydes and ketones provides the most straightforward route to valuable α-hydroxyamides. However, due to low stability of cyanohydrins and deactivation of the catalysts氰醇(α-羟基腈)是一种特殊类型的腈,很容易分解成氰化氢 (HCN) 和相应的羰基化合物。可通过醛和酮的氰化轻松获得的氰醇的水合提供了获得有价值的 α-羟基酰胺的最直接途径。然而,由于氰醇的低稳定性和释放的 HCN 使催化剂失活,氰醇的催化直接水合仍然在很大程度上未解决。一般来说,含有较大取代基的氰醇,如α,α-二芳基氰醇,降解速度更快,因此更难水合。在这里,我们报告了对各种氰醇的水合表现出高反应性的阳离子铂催化剂的开发。2 OH)X(OTf) 揭示了一个催化循环,包括形成五元金属环中间体,然后通过 H 2 O攻击二级氧化膦 (PR 2 OH) 配体的磷进行水解。我们发现 Pt 催化剂甲轴承富电子,适当小的咬角双膦配体提供了一种用于氰醇的水合反应性超级。由A催化的水合反应在环境温度下进行,并与多种氰醇一起发生,包括最难的 α,α-二芳基氰醇,具有良好的转化率。
-
METHOD FOR PRODUCING A CARBOXYLIC ACID AMIDE FROM A CARBONYL COMPOUND AND HYDROCYANIC ACID申请人:May Alexander公开号:US20110306784A1公开(公告)日:2011-12-15The invention relates to a method for producing a carboxylic acid amide from a carbonyl compound and hydrocyanic acid, comprising the steps of A) reacting a carbonyl compound with hydrocyanic acid to produce a hydroxycarboxylic acid nitrile, B) hydrolysis of the hydroxycarboxylic acid nitrile obtained in step A) in the presence of a catalyst comprising manganese dioxide, wherein a molar excess of carbonyl compound is used in relation to the hydrocyanic acid to react the carbonyl compound with hydrocyanic acid according to step A), and the reaction mixture obtained in step A) is not purified by distillation before the hydrolysis according to step B) is carried out. The invention furthermore relates to a method for producing alkyl(meth)acrylates from polymers, moulding compounds and moulded bodies, wherein a method for producing a carboxylic acid amide from a carbonyl compound and hydrocyanic acid is carried out in accordance with the method described above.
-
Process for producing alpha-hydroxycarboxylic acid amide申请人:Mitsubishi Gas Chemical Company, Inc.公开号:US04950801A1公开(公告)日:1990-08-21Disclosed is a process for producing .alpha.-hydroxy-carboxylic acid amide represented by the formula (I): ##STR1## wherein R.sup.1 and R.sup.2 are as defined in the specification, by a catalytic hydration reaction of cyanohydrin represented by the formula (II): ##STR2## which comprises using a modified manganese dioxide containing one or more of an alkali metal element and an alkaline earth element in an amount of 0.05 to 0.5 based on the manganese element in terms of atomic ratio.
-
METHOD FOR PRODUCING A-HYDROXYISOBUTYRIC ACID AMIDE AND REACTOR申请人:MITSUBISHI GAS CHEMICAL COMPANY, INC.公开号:US20160145196A1公开(公告)日:2016-05-26The present invention provides a method for producing α-hydroxyisobutyric acid amide by hydration of acetone cyanohydrin under the presence of a catalyst composed mainly of manganese oxide using a reactor in which at least two reaction regions are connected in series, the method being characterized by comprising: a step (B) of cyclically supplying at least a portion of a reaction liquid withdrawn from at least one reaction region to a first reaction region (I) in the reactor; and a step (b1) of further cyclically supplying at least a portion of the reaction liquid withdrawn from at least one reaction region to at least one reaction region other than the first reaction region. The method is also characterized in that an oxidizing agent is supplied to at least one reaction region in the reactor.
-
Integrated method for producing methyl methacrylate and hydrogen cyanide申请人:Mitsubishi Gas Chemical Company, Inc.公开号:US06075162A1公开(公告)日:2000-06-13A method of producing methyl methacrylate comprises Step 1 of producing acetone cyanhydrin from hydrogen cyanide and acetone; Step 2 of producing .alpha.-hydroxyisobutyramide by hydrating acetone cyanhydrin; Step 3 of producing methyl .alpha.-hydroxyisobutyrate and ammonia by a reaction of .alpha.-hydroxyisobutyramide and methanol; Step 4 of producing methyl methacrylate by dehydrating methyl .alpha.-hydroxyisobutyrate; and Step 5 of producing hydrogen cyanide in vapor phase by reacting methanol and the ammonia obtained in Step 3 over a solid catalyst in the presence of molecular oxygen. By using methanol in the step 3, the conversion ratio of .alpha.-hydroxyisobutyramide into methyl .alpha.-hydroxyisobutyrate can be increased because the equilibrium of the reaction is easily sifted toward the product side by removing ammonia being produced from the reaction system. The use of methanol in the step 3 produces additional advantages of efficiently linking the steps to eliminate the steps for separation and purification, thereby reducing the production cost. Step 1: HCN+CH.sub.3 COCH.sub.3 .fwdarw.(CH.sub.3).sub.2 (OH)CN; Step 2: (CH.sub.3).sub.2 C(OH)CN+H.sub.2 O.fwdarw.(CH.sub.3).sub.2 (OH)CONH.sub.2 Step 3: (CH.sub.3).sub.2 C(OH)CONH.sub.2 +CH.sub.3 OH.fwdarw.(CH.sub.3).sub.2 (OH)CO.sub.2 CH.sub.3 +NH.sub.3 Step 4: (CH.sub.3).sub.2 C(OH)CO.sub.2 CH.sub.3 .fwdarw.(CH.sub.2).sub.2 .dbd.C(CH.sub.3)CO.sub.2 CH.sub.3 +H.sub.2 O Step 5: CH.sub.3 OH+NH.sub.3 +O.sub.2 .fwdarw.HCN+3H.sub.2 O
表征谱图
-
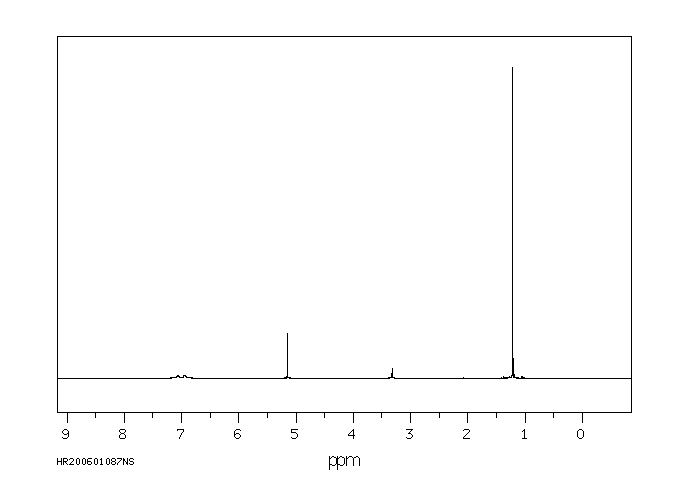
氢谱1HNMR
-
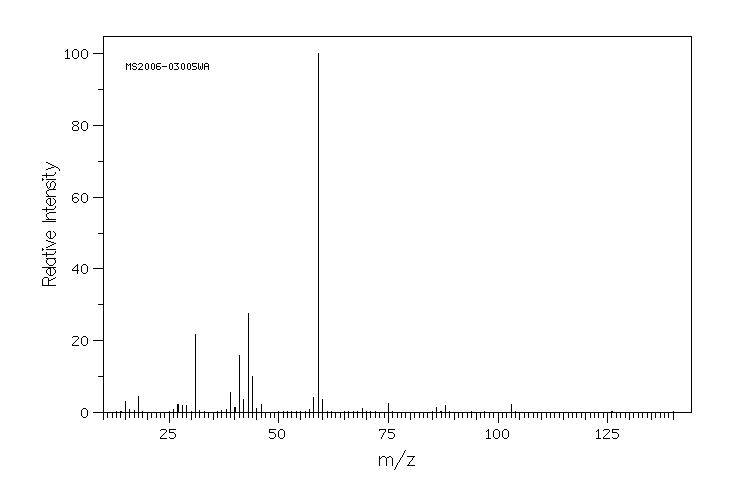
质谱MS
-
碳谱13CNMR
-
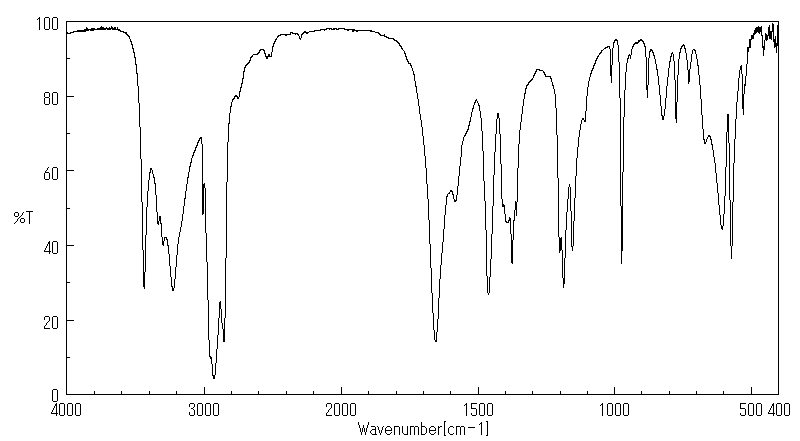
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷