3,3,5,5-四甲基环己醇 | 2650-40-0
物质功能分类
中文名称
3,3,5,5-四甲基环己醇
中文别名
——
英文名称
3,3,5,5-tetramethyl cyclohexanol
英文别名
3,3,5,5-tetramethylcyclohexyl alcohol;3,3,5,5-Tetramethylcyclohexanol;3,3,5,5-tetramethylcyclohexan-1-ol
CAS
2650-40-0
化学式
C10H20O
mdl
——
分子量
156.268
InChiKey
PIQXIIHPQBQVTP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:194.4±8.0 °C(Predicted)
-
密度:0.866±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2906199090
SDS
| Name: | 3 3 5 5-Tetramethylcyclohexanol 96% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 2650-40-0 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2650-40-0 | 3,3,5,5-Tetramethylcyclohexanol, 96% | 96 | 220-170-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2650-40-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 80.00 - 82.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H20O
Molecular Weight: 156.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2650-40-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,3,5,5-Tetramethylcyclohexanol, 96% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2650-40-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2650-40-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2650-40-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-(allyloxy)-1,1,3,3-tetramethylcyclohexane 1256151-39-9 C13H24O 196.333
反应信息
-
作为反应物:描述:参考文献:名称:对反应速率的立体效应II:双环醇氧化的速率和平衡常数摘要:在Meerwein-Ponndorf条件下,已确定了一系列双环醇与环己酮的氧化平衡常数。数据为解释醇氧化和酮还原的机理提供了热力学背景。平衡(ΔG的自由能牛)与由分子力学计算的值进行比较。DOI:10.1016/0040-4039(81)80131-1
-
作为产物:描述:3,3,5,5-四甲基环己酮 在 sodium tetrahydroborate 、 silica gel 作用下, 反应 10.0h, 以88%的产率得到3,3,5,5-四甲基环己醇参考文献:名称:探索催化剂功能——手性聚硼酸盐阴离子催化剂的电子调制摘要:VAPOL 和 VANOL 的硼酸配合物是一种手性阴离子平台,可作为不对称催化的多功能分级平台。该平台的结构基础是手性聚硼酸酯核心,它将醇(或酚)和拱形联芳基配体共价连接在一起。聚硼酸酯平台通过反应底物原位组装,因此可以由各种醇(或酚)和双酚配体快速组装出多重手性催化剂,用于筛选催化剂活性。在本研究中,探讨了硼氧酸酯催化剂中苯酚/醇组分的空间和电子性质的变化,以揭示它们对催化不对称氮丙啶化反应中不对称诱导的影响。哈米特的研究与两种底物在对映步骤中氢键结合到硼氧酸酯核心的机制是一致的。Hammett 研究的结果得到了一项计算研究的支持,其中发现,与阴离子硼氧酸盐核心键合的质子化亚胺氢的 H-O 距离随着并入阴离子硼氧酸盐核心的苯酚单元的电子释放能力的增加而减小。硼酸。结果与硼氧酸酯催化剂充当路易斯酸并通过路易斯酸/路易斯碱相互作用活化亚胺的机制不一致。Hammett 研究的结果得到了一项计算DOI:10.1021/acs.joc.1c01769
文献信息
-
[EN] 5-O-SUBSTITUTED 3-N-PHENYL-1,3,4-OXADIAZOLONES FOR MEDICAL USE<br/>[FR] 3-N-PHÉNYL-1,3,4-OXADIAZOLONES 5-O-SUBSTITUÉES POUR UNE UTILISATION MÉDICALE申请人:BIAL PORTELA & COMPANHIA S A公开号:WO2009084970A1公开(公告)日:2009-07-09The present invention relates to compounds having a 5-O-substituted 3-N-phenyl-1,3,4-oxadiazolone structural unit which have unexpectedly high level of inhibition of FAAH (fatty acid amide hydrolase). (I)
-
Method for the Direct Enantioselective Synthesis of Chiral Primary α-Amino Ketones by Catalytic α-Amination作者:Yixin Han、E. J. CoreyDOI:10.1021/acs.orglett.8b03733日期:2019.1.4A useful catalytic enantioselective approach has been developed for the synthesis of chiral ketamine analogs using Rh(II)-catalyzed amination of triisopropylsilyl enol ethers to form α-amino ketones with O-(4-nitrophenyl)hydroxylamine as nitrogen donor in 81–91% ee.
-
NOVEL 3-HYDROXY-5-ARYLISOTHIAZOLE DERIVATIVE申请人:Okano Akihiro公开号:US20120157459A1公开(公告)日:2012-06-21[Problem] To provide a GPR40 activating agent having, as an active ingredient, a novel compound having a GPR40 agonist action, a salt of the compound, a solvate of the salt or the compound, or the like, particularly, an insulin secretagogue and a prophylactic and/or therapeutic agent against diabetes, obesity, or other diseases. [Means of solving the problem] A compound of Formula (I): (where n is 0 to 2; p is 0 to 4; j is 0 to 3; k is 0 to 2; a ring A is an aryl group which is optionally substituted with L or a heterocyclic group which is optionally substituted with L; a ring B is a benzene ring, a pyridine ring, or a pyrimidine ring; X is O, S, —NR 7 —; and R 1 to R 7 are specific groups), a salt of the compound, or a solvate of the salt or the compound.
-
Use of substituted 3-phenyl-5-alkoxy-3H-(1,3,4)-oxadizol-2-ones for inhibiting pancreatic lipase申请人:——公开号:US20030236288A1公开(公告)日:2003-12-25The invention relates to a method for inhibiting pancreatic lipase, or the prophylaxis or treatment of obesity or diabetes mellitus of type 1 and 2, in a patient in need thereof, comprising administering to the patient a pharmaceutically effective amount of substituted 3-phenyl-5-alkoxy-3H-(1,3,4)-oxadiazol-2-ones of formula 1: 1 wherein R 1 , R 2 , R 3 , R 4 and R 5 are as defined herein, or a prodrug, solvate, pharmacologically acceptable salt or acid addition salt thereof.
-
[EN] COMPOUNDS HAVING A FUNGICIDAL ACTIVITY, THEIR AGRONOMIC COMPOSITIONS AND USE THEREOF FOR THE CONTROL OF PHYTOPATHOGENIC FUNGI<br/>[FR] COMPOSÉS AYANT UNE ACTIVITÉ FONGICIDE, LEURS COMPOSITIONS AGRONOMIQUES ET LEUR UTILISATION POUR LUTTER CONTRE DES CHAMPIGNONS PHYTOPATHOGÈNES申请人:ISAGRO SPA公开号:WO2020225700A1公开(公告)日:2020-11-12Compounds having general formula (I) with a high fungicidal activity and their use for the control of phytopathogenic fungi of important agricultural crops, are described.具有通式(I)的化合物具有很高的杀真菌活性,并且它们用于控制重要农作物的植物病原真菌。
表征谱图
-
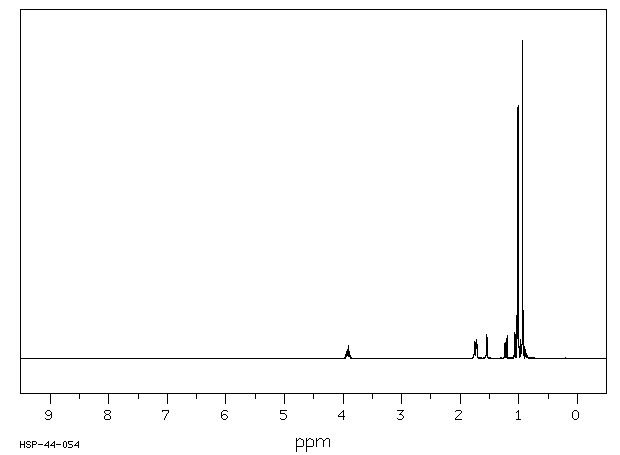
氢谱1HNMR
-
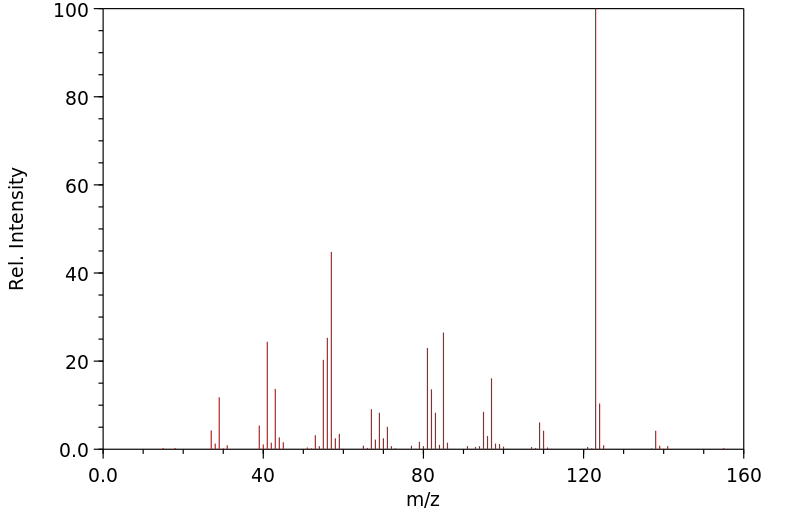
质谱MS
-
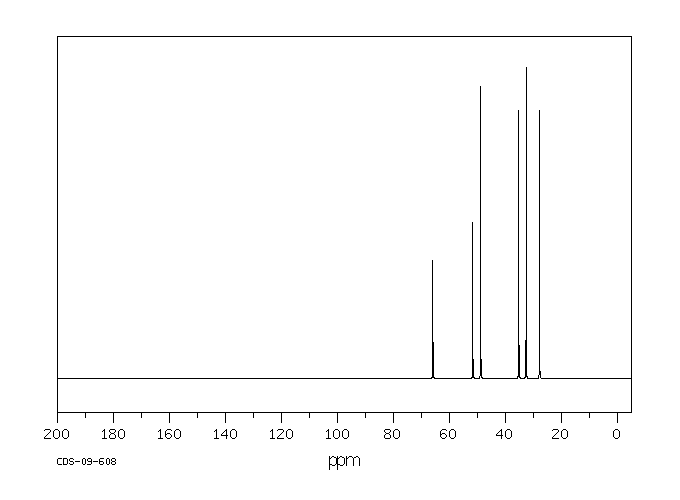
碳谱13CNMR
-
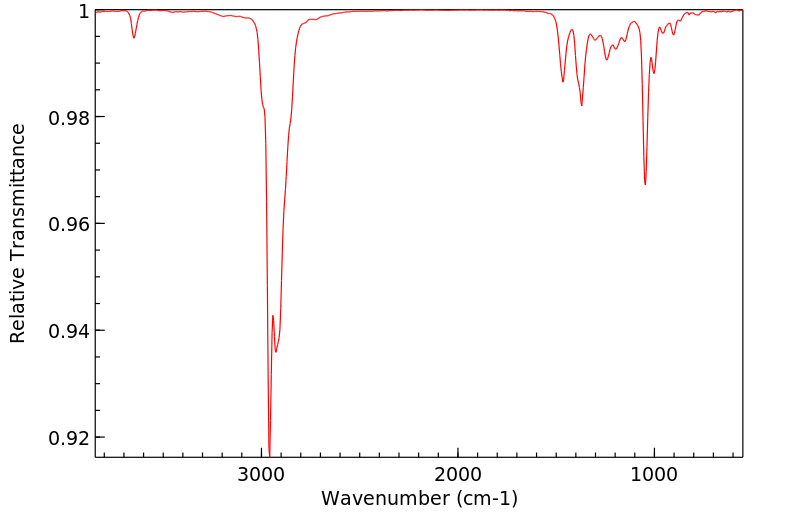
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷