3-溴-4-甲氧基苯甲醛 | 34841-06-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:51-54 °C (lit.)
-
沸点:108-110°C 1mm
-
密度:1.5313 (rough estimate)
-
闪点:108-110°C/1mm
-
溶解度:可溶于氯仿、DMSO、乙酸乙酯
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未有已知危险反应。请避免接触氧化物和碱。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT, AIR SENSITIVE
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:29130000
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封保存,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
: 3-Bromo-4-methoxybenzaldehyde
化学品俗名或商品名
1.2 鉴别的其他方法
3-Bromo-p-anisaldehyde
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 3-Bromo-p-anisaldehyde
别名
: C8H7BrO2
分子式
: 215.04 g/mol
分子量
成分 浓度
3-Bromo-p-anisaldehyde
-
化学文摘编号(CAS No.) 34841-06-0
EC-编号 252-241-8
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 溴化氢气
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 51 - 54 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
应用领域中提到的3-溴-4-甲氧基苯甲醛是一种医药中间体,它可以用于合成异香兰素和氯比普兰。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-溴-4-甲氧基甲苯 2-bromo-4-methylanisole 22002-45-5 C8H9BrO 201.063 3-溴-4-羟基苯甲醛 3-bromo-4-hydroxybenzylaldehyde 2973-78-6 C7H5BrO2 201.019 3-溴-4-甲氧基苄醇 (3-bromo-4-methoxy-phenyl)-methanol 38493-59-3 C8H9BrO2 217.062 3-溴-4-甲氧基苯甲酸甲酯 methyl 3-bromo-4-methoxybenzoate 35450-37-4 C9H9BrO3 245.073 —— 2-bromo-1-methoxy-4-propylbenzene 101538-30-1 C10H13BrO 229.117 5-溴水杨醛 5-bromosalicyclaldehyde 1761-61-1 C7H5BrO2 201.019 2-(3-溴-4-甲氧基苯基)-1,3-二氧戊环 2-(3-bromo-4-methoxyphenyl)-1,3-dioxolane 223418-72-2 C10H11BrO3 259.1 4-甲氧基苯甲醛 4-methoxy-benzaldehyde 123-11-5 C8H8O2 136.15 —— 2-(3-bromo-4-methoxy-phenyl)-[1,3]dithiane —— C11H13BrOS2 305.26 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-bromo-4-(methoxymethoxy)benzaldehyde 162269-90-1 C9H9BrO3 245.073 3-溴-4-甲氧基苯甲酸 3-bromo-4-methoxybenzoic acid 99-58-1 C8H7BrO3 231.046 3-溴-4-羟基苯甲醛 3-bromo-4-hydroxybenzylaldehyde 2973-78-6 C7H5BrO2 201.019 —— 2-bromo-1-methoxy-4-vinylbenzene 34039-30-0 C9H9BrO 213.074 5-溴-2-羟基-4-甲氧基苯甲醛 5-bromo-2-hydroxy-4-methoxybenzaldehyde 57543-36-9 C8H7BrO3 231.046 2-溴-4-(溴甲基)-1-甲氧基苯 2-bromo-4-(bromomethyl)-1-methoxybenzene 89978-72-3 C8H8Br2O 279.959 3-溴-4-甲氧基苄醇 (3-bromo-4-methoxy-phenyl)-methanol 38493-59-3 C8H9BrO2 217.062 2-溴-4-氯甲基-1-甲氧基苯 3-bromo-4-methoxybenzyl chloride 701-94-0 C8H8BrClO 235.508 3-溴-4-甲氧基苯甲酸甲酯 methyl 3-bromo-4-methoxybenzoate 35450-37-4 C9H9BrO3 245.073 3-溴-4-甲氧基苯甲腈 3-bromo-4-methoxybenzonitrile 117572-79-9 C8H6BrNO 212.046 —— 2-(3-bromo-4-methoxyphenyl)-2-oxoacetaldehyde —— C9H7BrO3 243.057 —— 2-bromo-4-ethynyl-1-methoxybenzene 859211-28-2 C9H7BrO 211.058 3-溴-4-甲氧基苄胺 (3-bromo-4-methoxyphenyl)methanamine 247254-47-3 C8H10BrNO 216.077 —— 3-bromo-4-cyclohexylmethyloxy Benzaldehyde 222627-68-1 C14H17BrO2 297.192 —— (E)-2-bromo-1-methoxy-4-styrylbenzene —— C15H13BrO 289.172 5-烯丙基-2-甲氧基溴苯 2-bromo-4-allylanisole 87688-94-6 C10H11BrO 227.101 3-溴-4-甲氧基苯乙腈 2-(3-bromo-4-methoxyphenyl)acetonitrile 772-59-8 C9H8BrNO 226.073 —— (3-bromo-4-methoxy-benzyl)-methyl-amine 645378-58-1 C9H12BrNO 230.104 (3-溴-4-甲氧基苯基)(苯基)甲烷 (3-bromo-4-methoxyphenyl)(phenyl)methane 1027513-90-1 C14H13BrO 277.161 3-(3-溴-4-甲氧基苯基)丙腈 3-(3-bromo-4-methoxyphenyl)propanenitrile 943-66-8 C10H10BrNO 240.099 —— 1-(3-bromo-4-methoxyphenyl)-N,N-dimethylmethanamine 774-82-3 C10H14BrNO 244.131 —— (E)-4-(3-Bromo-4-methoxy-phenyl)-but-3-en-2-one 1331560-65-6 C11H11BrO2 255.111 1-(3-溴-4-甲氧基苯基)乙醇 3-bromo-α-methyl-4-methoxybenzenemethanol 94670-25-4 C9H11BrO2 231.089 —— (E)-3-(3-bromo-4-methoxy-phenyl)prop-2-enoic acid 1080-07-5 C10H9BrO3 257.084 3-溴-4-甲氧基肉桂酸 3-(3-bromo-4-methoxyphenyl)acrylic acid 1080-07-5 C10H9BrO3 257.084 3-溴-4-甲氧基苯乙酸 (3-bromo-4-methoxy-phenyl)-acetic acid 774-81-2 C9H9BrO3 245.073 —— 6-(3-bromo-4-methoxyphenyl)-hexan-2-one 936630-72-7 C13H17BrO2 285.181 —— (E)-2-bromo-1-methoxy-4-(2-nitrovinyl)benzene 70400-19-0 C9H8BrNO3 258.071 —— 1-methoxy-2-bromo-4-(2-nitro-vinyl)-benzene 70400-19-0 C9H8BrNO3 258.071 ((3-溴-4-甲氧基苯基)亚甲基)甲烷-1,1-二甲腈 2-[(3-Bromo-4-methoxyphenyl)methylidene]propanedinitrile 313670-97-2 C11H7BrN2O 263.093 —— (E)-methyl 3-(3-bromo-4-methoxyphenyl)acrylate 888738-40-7 C11H11BrO3 271.111 —— 2-bromo-4-(4-fluorobenzyl)-1-methoxybenzene 1158234-81-1 C14H12BrFO 295.151 —— 2-(3-bromo-4-methoxybenzylidene)hydrazinecarboxamide —— C9H10BrN3O2 272.101 —— 3-bromo-4-methoxybenzaldehyde thiosemicarbazone 306279-93-6 C9H10BrN3OS 288.168 —— ethyl (E)-3-(3-bromo-4-methoxyphenyl)acrylate 540778-94-7 C12H13BrO3 285.137 —— (E)-3-(3-bromo-4-methoxyphenyl)-1-phenyl-2-propen-1-one —— C16H13BrO2 317.182 —— (E)-3-(3-bromo-4-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one 1437742-78-3 C16H13BrO3 333.181 —— (S)-1-(3-Bromo-4-methoxy-phenyl)-ethane-1,2-diol 395639-54-0 C9H11BrO3 247.089 2-(3-溴-4-甲氧基苯基)-1,3-二氧戊环 2-(3-bromo-4-methoxyphenyl)-1,3-dioxolane 223418-72-2 C10H11BrO3 259.1 —— 1-(3-bromo-4-methoxyphenyl)-7-(4-hydroxyphenyl)heptan-3-one 936630-82-9 C20H23BrO3 391.305 3-溴-4-甲氧基-苯甲酸苯胺 3-bromo-4-methoxy-benzoic acid anilide 356541-58-7 C14H12BrNO2 306.159 4-甲氧基苯甲醛 4-methoxy-benzaldehyde 123-11-5 C8H8O2 136.15 —— 3-Brom-4-methoxy-N-phenylbenzylamin 67342-73-8 C14H14BrNO 292.175 —— (E)-3'-bromo-3,4',5-trimethoxystilbene —— C17H17BrO3 349.224 —— ethyl 2-((3-bromo-4-methoxybenzyl)amino)acetate —— C12H16BrNO3 302.168 —— 3-bromo-4-methoxystyrene oxide 258267-66-2 C9H9BrO2 229.073 —— (S)-2-(3-Bromo-4-methoxy-phenyl)-oxirane 395639-55-1 C9H9BrO2 229.073 —— 3-(3-bromo-4-methoxyphenyl)-2-oxopropanoic acid 29974-00-3 C10H9BrO4 273.083 —— 2-bromo-4-(1-bromo-2,2-difluoroethyl)-1-methoxybenzene —— C9H8Br2F2O 329.967 —— 2-(3-bromo-4-methoxyphenyl)-1,3-dioxane 208399-74-0 C11H13BrO3 273.126 —— ((3-bromo-4-methoxybenzyl)oxy)(tert-butyl)-dimethylsilane 905101-08-8 C14H23BrO2Si 331.325 —— α-amino-(3-bromo-4-methoxy-phenyl)acetonitrile —— C9H9BrN2O 241.087 —— 1-(3-Bromo-4-methoxy-phenyl)-but-3-en-1-ol 204846-45-7 C11H13BrO2 257.127 —— (3-Bromo-4-methoxyphenyl)-(4-ethoxyphenyl)methanol 1221506-22-4 C16H17BrO3 337.213 —— 1-(3-bromo-4-methoxyphenyl)-2-nitropropene 31558-30-2 C10H10BrNO3 272.098 —— 2-(3-bromo-4-methoxy-phenyl)-[1,3]dithiane —— C11H13BrOS2 305.26 —— (E)-3-(3-bromo-4-methoxyphenyl)-1-(3-hydroxyphenyl)prop-2-en-1-one 1391150-70-1 C16H13BrO3 333.181 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:参考文献:名称:一种异香兰素的合成新工艺摘要:本发明公开了一种异香兰素的合成新工艺,涉及有机合成技术领域,以4‑羟基苯甲醛为原料,先与溴素反应得到3‑溴‑4‑羟基苯甲醛,再与碘甲烷反应得到3‑溴‑4‑甲氧基苯甲醛,然后在氢氧化钠与氯化亚铜作用下经水解制得异香兰素。本发明以价廉易得的4‑羟基苯甲醛为起始原料,通过温和的反应条件制得异香兰素,在降低成本的同时提高工艺的经济效益;通过最优工艺制得的产物异香兰素总收率达到64.1%,显著提高了各步原料的转化率,适用于工业化生产。公开号:CN107118087A
-
作为产物:描述:4-甲氧基苯甲醛 在 bismuth(III) chloride 、 N-溴代丁二酰亚胺(NBS) 、 D(+)-10-樟脑磺酸 作用下, 以 乙腈 为溶剂, 以95 %的产率得到3-溴-4-甲氧基苯甲醛参考文献:名称:D-樟脑磺酸-BiCl3 催化 N-卤代琥珀酰亚胺 (NXS) 卤化芳香族化合物摘要:空气条件下,0.5~10 mol% D-樟脑磺酸-BiCl3 与N-溴代琥珀酰亚胺 (NBS) 在 MeCN 中进行芳香溴化反应,并扩展到N-氯代琥珀酰亚胺 (NCS) 和N-溴代琥珀酰亚胺 (NCS) 的反应。碘代琥珀酰亚胺(NIS)。还尝试了一些药物和天然产物的卤化。在不除去溶剂的情况下,实现了一锅溴化/Suzuki-Miyaura交叉偶联和溴化/Sonogashira偶联反应。DOI:10.1039/d4ob00837e
文献信息
-
Ring strain and total syntheses of modified macrocycles of the isoplagiochin type作者:Andreas Speicher、Timo Backes、Kerstin Hesidens、Jürgen KolzDOI:10.3762/bjoc.5.71日期:——
Macrocycles of the bisbibenzyl-type are natural products that are found exclusively in bryophytes (liverworts). The molecular framework of the subtype “isoplagiochin” is of substantial structural interest because of the chirality of the entire molecule, which arises from two biaryl axes in combination with two helical two-carbon units in a cyclic arrangement. From a structural as well as a synthetic point of view we report on the total synthesis of compounds which possess more rigid two-carbon biaryl bridges like stilbene (
E orZ ) or even tolane moieties which were introduced starting with a Sonogashira protocol. The McMurry method proved to be a powerful tool for the cyclization to these considerably ring-strained macrocycles. -
Oxime derivatives for the treatment of dyslipidemia and hypercholesteremia申请人:——公开号:US20030083357A1公开(公告)日:2003-05-01The present invention relates to compounds of Formula (I) which may be useful in the treatment of diseases, such as, metabolic disorders, dyslipidemia and/or hyperchloesterolemia: 1本发明涉及Formula (I)的化合物,可能在治疗疾病,如代谢紊乱、血脂异常和/或高胆固醇血症方面有用:
-
Heterocyclic derivatives for the treatment of diabetes and other diseases申请人:Maxia Pharmaceuticals, Inc.公开号:US06515003B1公开(公告)日:2003-02-04The present invention relates to certain substituted heterocycles of Formula (I) which are useful in the treatment of diseases related to lipid and carbohydrate metabolism, such as type 2 diabetes, adipocyte differentiation, uncontrolled proliferation, such as lymphoma, Hodgkin's Disease, leukemia, breast cancer, prostate cancer or cancers in general; and inflammation, such as osteoarthritis, rheumatoid arthritis, Crohn's Disease or Inflammatory Bowel Disease.
-
Linear diarylheptanoids as potential anticancer therapeutics: synthesis, biological evaluation, and structure–activity relationship studies作者:A. F. M. Motiur Rahman、Yang Lu、Hwa-Jong Lee、Hyunji Jo、Wencui Yin、Mohammad Sayed Alam、Hyochang Cha、Adnan A. Kadi、Youngjoo Kwon、Yurngdong JahngDOI:10.1007/s12272-018-1004-8日期:2018.12detrimental action of the drug toward the intestinal flora, a series of linear diarylheptanoids (LDHs) were designed and synthesized in 7 steps with good-to-moderate yields. All synthesized compounds were evaluated for their antibacterial, antiproliferative, and topoisomerase-I and -IIα inhibitory activity. Overall, all compounds showed little to no activity against the bacterial strains tested. Most of the为了开发有效的抗癌疗法,对癌细胞具有更大的选择性并减少副作用,例如由于药物对肠道菌群的有害作用引起的催吐作用,设计并分 7 个步骤合成了一系列线性二芳基庚烷 (LDH)产量良好至中等。评估了所有合成化合物的抗菌、抗增殖和拓扑异构酶-I 和-IIα 抑制活性。总体而言,所有化合物对所测试的细菌菌株几乎没有活性。大多数合成的化合物对人乳腺癌细胞系(T47D)显示出良好的抗增殖活性;具体而言,化合物 6a、6d、7j 和 7e 的 IC50 值分别为 0.09、0.64、0.67 和 0.99 μM。在测试的化合物中,7b 抑制了 topo-I 9。在 100 μM 的浓度下,3%(喜树碱 68.8%)、7e 和 7h 分别抑制了 38.4% 和 47.4%(依托泊苷 76.9%)的 topo-IIα。这些结果表明,可以通过减少对不同微生物的抑制作用来提高对癌细胞的选择性,从而获得一组有前景的抗癌药物。
-
Total synthesis, antiprotozoal and cytotoxicity activities of rhuschalcone VI and analogs作者:Shetonde O. Mihigo、Wendimagegn Mammo、Merhatibeb Bezabih、Kerstin Andrae-Marobela、Berhanu M. AbegazDOI:10.1016/j.bmc.2010.02.055日期:2010.4The total synthesis of a potent antiplasmodial natural bichalcone, rhuschalcone VI, is described starting from simple and available resorcinol and 4-hydroxybenzaldehyde. Key steps include the solvent-free Aldol syntheses of chalcones, and the successful application of the Suzuki–Miyaura coupling reaction in the synthesis of bichalcones. The present work constitutes a general method for the rapid syntheses
表征谱图
-
氢谱1HNMR
-
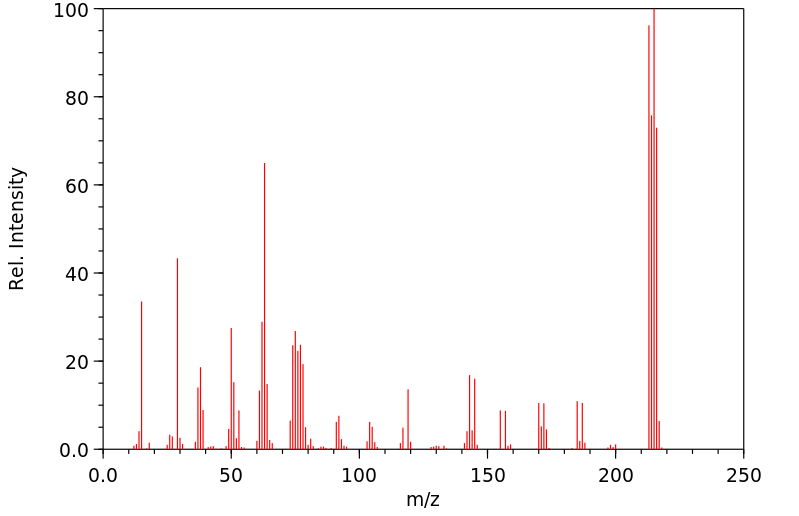
质谱MS
-
碳谱13CNMR
-
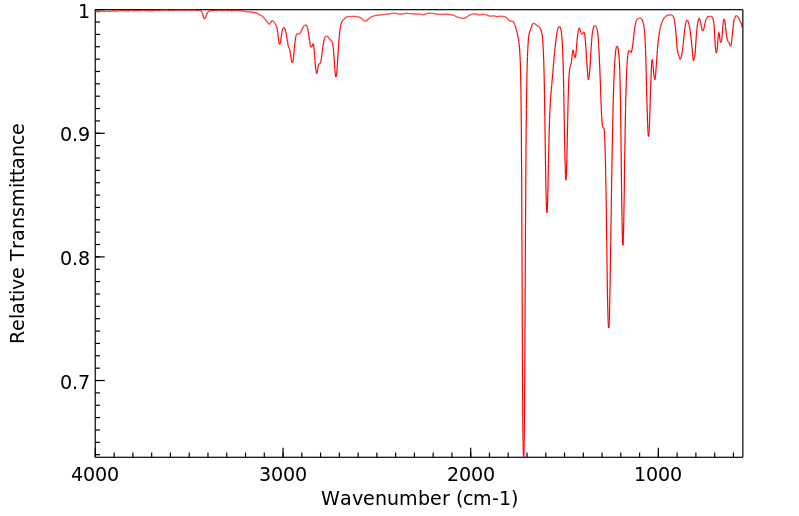
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息