3-硝基甲苯 | 99-08-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:15 °C
-
沸点:230-231 °C(lit.)
-
密度:1.157 g/mL at 25 °C(lit.)
-
蒸气密度:4.73 (vs air)
-
闪点:215 °F
-
溶解度:水中的溶解度0.419克/升
-
介电常数:23.800000000000001
-
暴露限值:NIOSH REL: TWA 2 ppm (11 mg/m3), IDLH 200 ppm; OSHA PEL: TWA 5 ppm (30 mg/m3); ACGIH TLV: TWA 2 ppm (adopted).
-
LogP:2.4
-
物理描述:M-nitrotoluene appears as yellow crystals that melt at 59°F to a yellow liquid. Often therefore encountered as a liquid. Flash point 223°F. Boiling point 450°F. Insoluble in water. Toxic by inhalation and ingestion.
-
颜色/状态:Yellow liquid [Note: A solid below 59 degrees F]
-
气味:Weak, aromatic odor
-
蒸汽密度:4.73 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.107 mm Hg at 25 °C
-
亨利常数:9.30e-06 atm-m3/mole
-
大气OH速率常数:9.50e-13 cm3/molecule*sec
-
稳定性/保质期:
- 稳定性:稳定。
- 禁配物:强氧化剂、强还原剂、强碱。
- 应避免接触的条件:受热。
- 聚合危害:不聚合。
- 分解产物:氮氧化物。
-
分解:The substance decomposes on burning producing toxic gases, including carbon monoxide and nitrogen oxides.
-
粘度:2.33 cP at 20 °C
-
燃烧热:11,232 Btu/lb = 86.3 cal/g = 3.61X10+5 J/kg
-
汽化热:11,831.1 gcal/gmole
-
表面张力:43.5 dynes/cm at 25 °C
-
电离电位:9.48 eV
-
气味阈值:1.74 ppm
-
折光率:Index of refraction: 1.5466 at 10 °C
-
保留指数:1192.7
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 2 ppm (11 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S16,S27,S28A,S36/37,S37,S45,S61
-
危险类别码:R23/24/25,R51/53,R36/37/38,R33
-
WGK Germany:2
-
海关编码:29042000
-
危险品运输编号:UN 1664 6.1/PG 2
-
危险类别:6.1
-
RTECS号:XT2975000
-
包装等级:II
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不超过35℃,相对湿度不超过80%。 - 包装密封,并与氧化剂、还原剂、碱类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有合适的材料收容泄漏物。
制备方法与用途
3-硝基甲苯由甲苯在50℃以下用混酸硝化后,经分馏和精制而得。甲苯硝化的产物多样,包括邻硝基甲苯、对硝基甲苯、间硝基甲苯、2,4—二硝基甲苯和2,4,6—三硝基甲苯等。硝基甲苯及其二硝基衍生物是重要的医药、染料及农药中间体。在常规条件下,邻位产物多于对位,而对位的产量又远高于间位。当前国内市场对面、对位的硝基甲苯需求较大,因此国内外研究者致力于提高其收率。尽管已有一定进展,但生成一定程度的间硝基甲苯仍是不可避免的。
性质3-硝基甲苯为淡黄色油状液体,具有类似硝基苯的味道。它相对密度1.1571,熔点为16℃,沸点在230~231℃之间。几乎不溶于水,但能溶解于乙醇、乙醚、氯仿和苯中,并且可以与水蒸气一同挥发。
制备间硝基甲苯可通过甲苯在50℃以下用混酸硝化后,经分馏和精制而获得。
应用由间硝基甲苯合成下游产品主要是苯环上的两个官能团进行氧化、还原或氯化反应。
化学性质3-硝基甲苯为黄色易燃液体或晶体。不溶于水,可溶于苯,并且与乙醇和乙醚混溶。
用途主要用于有机合成领域,作为农药、染料、医药、彩色显影剂、塑料、合成纤维及助剂的中间体。
生产方法在工业生产邻硝基甲苯和对硝基甲苯时,可同时回收间硝基甲苯。由3-硝基-4-氨基甲苯经一系列过程可制备间硝基甲苯,收率约为67%。
毒性分级高毒
急性毒性- 大鼠口服 LD50: 1072 毫克/公斤
- 小鼠口服 LD50: 330 毫克/公斤
与空气混合可爆。
可燃性危险特性遇明火可燃;燃烧时产生有毒氮氧化物烟雾。
储运特性库房应保持通风、低温和干燥,并与其他氧化剂或食品添加剂分开存放。
灭火剂 职业标准- 时间加权平均容许浓度(TWA):29 毫克/立方米
- 短时间接触容许浓度(STEL):58 毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 间硝基氯化苄 3-Nitrobenzyl chloride 619-23-8 C7H6ClNO2 171.583 3-硝基溴苄 1-bromomethyl-3-nitrobenzene 3958-57-4 C7H6BrNO2 216.034 4-硝基-2-甲苯胺 2-methyl-4-nitro-benzenamine 99-52-5 C7H8N2O2 152.153 3-硝基苯乙腈 (3-nitrophenyl)acetonitrile 621-50-1 C8H6N2O2 162.148 3-硝基苯乙醛 2-(3-nitrophenyl)acetaldehyde 66146-33-6 C8H7NO3 165.148 3-硝基苯乙醇 2-(3-nitrophenyl)ethanol 52022-77-2 C8H9NO3 167.164 1-(重氮甲基)-3-硝基苯 (m-nitrophenyl)diazomethane 19479-81-3 C7H5N3O2 163.136 2-碘-5-硝基甲苯 2-iodo-5-nitrotoluene 5326-38-5 C7H6INO2 263.035 4-甲基-2-硝基苯胺 4-methyl-2-nitroaniline 89-62-3 C7H8N2O2 152.153 3-亚硝基甲苯 3-nitrosotoluene 620-26-8 C7H7NO 121.139 3-硝基邻二甲苯 2,3-dimethylnitrobenzene 83-41-0 C8H9NO2 151.165 AR-甲基-AR-硝基苯胺 2-Methyl-6-nitroaniline 570-24-1 C7H8N2O2 152.153 3-硝基苯乙酸 3-nitro-benzeneacetic acid 1877-73-2 C8H7NO4 181.148 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 —— m-nitrobenzylphosphonic acid 40299-58-9 C7H8NO5P 217.118 —— 3-nitrobenzyl phenyl sulfide 80239-81-2 C13H11NO2S 245.302 2-甲基-4-硝基苯甲酸 2-methyl-4-nitrobenzoic acid 1975-51-5 C8H7NO4 181.148 1-(溴甲基)-6-甲基-2-硝基苯 2-(bromomethyl)-3-methyl-1-nitrobenzene 77378-54-2 C8H8BrNO2 230.061 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 间硝基苯腈 3-nitrobenzonitrile 619-24-9 C7H4N2O2 148.121 —— 1-(fluoromethyl)-3-nitrobenzene 455-94-7 C7H6FNO2 155.129 间硝基苯甲醛 3-nitro-benzaldehyde 99-61-6 C7H5NO3 151.122 间硝基氯化苄 3-Nitrobenzyl chloride 619-23-8 C7H6ClNO2 171.583 3-硝基溴苄 1-bromomethyl-3-nitrobenzene 3958-57-4 C7H6BrNO2 216.034 4-硝基-2-甲苯胺 2-methyl-4-nitro-benzenamine 99-52-5 C7H8N2O2 152.153 3,5-二硝基甲苯 3,5-dinitrotoluene 618-85-9 C7H6N2O4 182.136 —— 3-nitrobenzaldoxime 3431-62-7 C7H6N2O3 166.136 3-硝基苯基二氯甲烷 m-nitrobenzylidene chloride 619-28-3 C7H5Cl2NO2 206.028 —— 1-(dibromomethyl)-3-nitrobenzene 70970-06-8 C7H5Br2NO2 294.93 3-硝基苯乙醇 2-(3-nitrophenyl)ethanol 52022-77-2 C8H9NO3 167.164 2-甲基-4-硝基苯酚 2-methyl-4-nitrophenol 99-53-6 C7H7NO3 153.137 4-甲基-2-硝基苯胺 4-methyl-2-nitroaniline 89-62-3 C7H8N2O2 152.153 2-氯-5-硝基甲苯 1-chloro-2-methyl-4-nitrobenzene 13290-74-9 C7H6ClNO2 171.583 2-氟-5-硝基甲苯 5-nitro-2-fluorotoluene 455-88-9 C7H6FNO2 155.129 2-溴-5-硝基甲苯 2-bromo-5-nitrotoluene 7149-70-4 C7H6BrNO2 216.034 间硝基三氟甲苯 3-trifluoromethylnitrobenzene 98-46-4 C7H4F3NO2 191.109 间甲苯基羟胺 N-(3-methylphenyl)hydroxylamine 620-25-7 C7H9NO 123.155 3-亚硝基甲苯 3-nitrosotoluene 620-26-8 C7H7NO 121.139 —— 1-azidomethyl-3-nitrobenzene 126799-84-6 C7H6N4O2 178.15 —— β-NO2C6H4CBr3 95111-05-0 C7H4Br3NO2 373.826 2,5-二硝基甲苯 2,5-dinitrotoluene 619-15-8 C7H6N2O4 182.136 3-硝基-4-氯甲苯 1-chloro-4-methyl-2-nitro-benzene 89-60-1 C7H6ClNO2 171.583 AR-甲基-AR-硝基苯胺 2-Methyl-6-nitroaniline 570-24-1 C7H8N2O2 152.153 4-溴-3-硝基甲苯 4-Bromo-3-nitrotoluene 5326-34-1 C7H6BrNO2 216.034 间硝基苯甲酸 3-nitrobenzoic acid 121-92-6 C7H5NO4 167.121 3-硝基苯乙酸 3-nitro-benzeneacetic acid 1877-73-2 C8H7NO4 181.148 3-硝基乙酸苄酯 3-nitrobenzyl acetate 21388-97-6 C9H9NO4 195.175 3-硝基苯甲酰胺 3-nitrobenzamide 645-09-0 C7H6N2O3 166.136 3,4-二硝基甲苯 3,4-Dinitrotoluene 610-39-9 C7H6N2O4 182.136 —— 2,2-dimethyl-3-(3-nitrophenyl)propanenitrile —— C11H12N2O2 204.228 2-氯-3-硝基甲苯 2-chloro-3-nitrotoluene 3970-40-9 C7H6ClNO2 171.583 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 2-甲基-6-硝基苯酚 2-methyl-6-nitrophenol 13073-29-5 C7H7NO3 153.137 —— 1-(3-nitrobenzylidene)-2-phenylhydrazine 7539-23-3 C13H11N3O2 241.249 —— N-ethyl-2-methyl-4-nitro-aniline 88374-25-8 C9H12N2O2 180.206 1-溴-2-(溴甲基)-4-硝基苯 1-bromo-2-(bromomethyl)-4-nitrobenzene 939-82-2 C7H5Br2NO2 294.93 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:Giua, Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, 1914, vol. <5> 23 II, p. 486摘要:DOI:
-
作为产物:参考文献:名称:取代的苯胺的有机催化氧化为乙氧基苯和硝基化合物:机理研究不包括二环氧乙烷中间体的参与摘要:开发了有机催化和环境友好的方法来选择性氧化取代的苯胺。利用2,2,2-三氟苯乙酮介导的氧化过程,可以将取代的苯胺转化为a氧基苯,同时进行简单的处理...DOI:10.1039/c6gc03174a
-
作为试剂:描述:参考文献:名称:Manske et al., Canadian Journal of Research, Section B: Chemical Sciences, 1942, vol. 20, p. 133,135摘要:DOI:
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
MACROCYCLIC COMPOUNDS AND THEIR USE AS KINASE INHIBITORS申请人:Combs Andrew Paul公开号:US20090286778A1公开(公告)日:2009-11-19The present invention relates to macrocyclic compounds of Formula I: or pharmaceutically acceptable salts thereof or quaternary ammonium salts thereof wherein constituent members are provided hereinwith, as well as their compositions and methods of use, which are JAK/ALK inhibitors useful in the treatment of JAK/ALK-associated diseases including, for example, inflammatory and autoimmune disorders, as well as cancer.
-
Benzene Sulfonamide Thiazole and Oxazole Compounds申请人:Adams Jerry Leroy公开号:US20090298815A1公开(公告)日:2009-12-03The present invention provides thiazole sulfonamide and oxazole sulfonamide compounds, compositions containing the same, as well as processes for the preparation and methods for their use as pharmaceutical agents.
-
Copper-Based Intermetallic Electride Catalyst for Chemoselective Hydrogenation Reactions作者:Tian-Nan Ye、Yangfan Lu、Jiang Li、Takuya Nakao、Hongsheng Yang、Tomofumi Tada、Masaaki Kitano、Hideo HosonoDOI:10.1021/jacs.7b08252日期:2017.11.29The development of transition metal intermetallic compounds, in which active sites are incorporated in lattice frameworks, has great potential for modulating the local structure and the electronic properties of active sites, and enhancing the catalytic activity and stability. Here we report that a new copper-based intermetallic electride catalyst, LaCu0.67Si1.33, in which Cu sites activated by anionic过渡金属间化合物的开发,其中活性位点并入晶格骨架中,具有很大的潜力来调节活性位点的局部结构和电子性质,并增强催化活性和稳定性。在这里,我们报道了一种新型的铜基金属间电催化剂LaCu 0.67 Si 1.33,其中具有低功函的阴离子电子激活的Cu位原子原子地分散在晶格骨架中,并提供硝基芳烃的选择性加氢,其营业额高40倍以上频率(TOF高达5084 h –1),而不是经过深入研究的金属负载催化剂。利用同位素效应的动力学分析表明,氢键的裂解是决定速率的步骤。出乎意料的是,LaCu 0.67 Si 1.33的高载流子密度和低逸出功(LWF)特性使得能够以极低的活化能(E a = 14.8 kJ·mol –1)活化氢分子。此外,LaCu 0.67 Si 1.33的高氧亲合力可实现通过硝基优先吸附硝基芳烃表面,导致高化学选择性。本发明的有效催化剂可以进一步引发具有高活性的其他含氧官能团例如醛和酮的氢化
-
Microwave-Assisted Rapid and efficient Reduction of Aromatic Nitro Compounds to Amines with Propan-2-ol over Nanosized Perovskite-type SmFeO<sub>3</sub> powder as a New Recyclable Heterogeneous Catalyst作者:Saeid Farhadi、Firouzeh Siadatnasab、Maryam KazemDOI:10.3184/174751911x12964930076647日期:2011.2
Nanosized perovskite-type SmFeO3 powder, prepared through the thermal decomposition of Sm[Fe(CN)6].4H2O with an average particle diameter of 28 nm and a specific surface area of 42 m2 g−1, was used as a recyclable heterogeneous catalyst for the efficient and selective reduction of aromatic nitro compounds into the corresponding amines by using propan-2-ol as a hydrogen donor (reducing agent) and KOH as a promoter under microwave irradiation. This highly regio- and chemoselective catalytic method is fast, clean, inexpensive, high yielding and also compatible with the substrates containing easily reducible functional groups. In addition, the nanosized SmFeO3 catalyst can be reused without loss of activity.
表征谱图
-
氢谱1HNMR
-
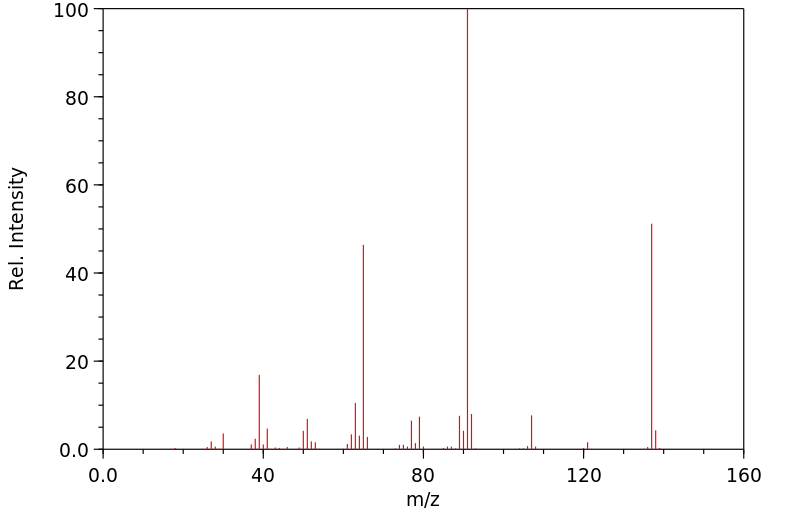
质谱MS
-
碳谱13CNMR
-
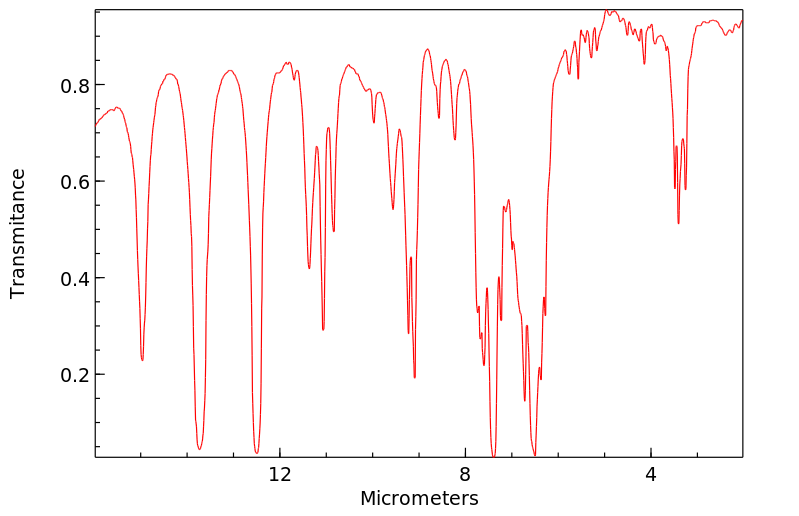
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息