3-羟基己酸 | 10191-24-9
物质功能分类
中文名称
3-羟基己酸
中文别名
——
英文名称
3-hydroxyhexanoic acid
英文别名
3(R,S)-hydroxyhexanoic acid;Acide hydroxy-3 hexanoique;3-Hydroxyhexanoate;n-butanolacetic acid;3-hydroxy-hexanoic acid;3-Hydroxy-capronsaeure
CAS
10191-24-9
化学式
C6H12O3
mdl
MFCD02259045
分子量
132.159
InChiKey
HPMGFDVTYHWBAG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:13 °C
-
沸点:259.6±23.0 °C(Predicted)
-
密度:1.100±0.06 g/cm3(Predicted)
-
溶解度:DMSO(少许)、甲醇(少许)、水(少许)
-
LogP:-0.080 (est)
-
稳定性/保质期:
存在于烟叶中。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2918199090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存储条件:2-8°C,干燥,密封。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-羟基己酸甲酯 methyl 3-hydroxyhexanoate 21188-58-9 C7H14O3 146.186 3-羟基己酸乙酯 3-hydroxy-hexanoic acid ethyl ester 2305-25-1 C8H16O3 160.213 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-3-hydroxyhexanoic acid 66997-60-2 C6H12O3 132.159 3-羟基己酸甲酯 methyl 3-hydroxyhexanoate 21188-58-9 C7H14O3 146.186 3-羟基己酸乙酯 3-hydroxy-hexanoic acid ethyl ester 2305-25-1 C8H16O3 160.213
反应信息
-
作为反应物:描述:3-羟基己酸 在 二甲基二环氧乙烷 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 作用下, 以 乙醇 、 丙酮 为溶剂, 生成 3-hydroxy-5-propyl-5H-furan-2-one参考文献:名称:Synthesis of α-Keto Esters and Amides via Oxidative Cleavage of Cyanoketophosphoranes by Dimethyldioxirane摘要:DOI:10.1021/jo0015974
-
作为产物:描述:参考文献:名称:Fittig; Baker, Justus Liebigs Annalen der Chemie, 1894, vol. 283, p. 117摘要:DOI:
文献信息
-
General synthetic routes to β-hydroxy-acids from t-butyl esters and the Reformatskii reaction作者:D. A. Cornforth、A. E. Opara、G. ReadDOI:10.1039/j39690002799日期:——modified Reformatskii procedure which leads directly to the hydroxy-acid. With highly hindered ketones or under conditions of inverse addition, self-condensation of the Reformatskii reagents from the t-butyl esters of α-bromopropionic acid and α-bromoisobutyric acid seriously reduce the yields of β-hydroxy-esters.
-
[EN] IONIC LIQUID SOLVENTS<br/>[FR] SOLVANTS LIQUIDES IONIQUES申请人:UNIV DUBLIN CITY公开号:WO2010097412A1公开(公告)日:2010-09-02A chiral ionic compound comprising an alkyl substituted imidazolium or pyridinium cationic core having an alkyl ester side chain (-alkyl-C(O)O-) directly linked to the core and an associated counter anion, characterized in that the -O- atom of the ester side chain is linked to an alpha, a beta or a gamma hydroxycarboxylic acid functionality via the alpha, beta or gamma hydroxy of the acid functionality and the hydroxycarboxylic acid functionality has at least one asymmetric carbon, or characterized in that an -N= atom of the alkyl substituted imidazolium or pyridinium cationic core is substituted with an alpha, a beta or a gamma hydroxy group of a alpha, a beta or a gamma hydroxycarboxylic acid functionality and the hydroxycarboxylic acid functionality has at least one asymmetric carbon. The chiral ionic liquids (CILs) may be used as novel solvents, in particular for organic synthesis. The CILs have the potential to induce asymmetry into substrates or catalysts in a variety of organic transformations. A number of the compounds have low antimicrobial and low antifungal toxicities and are also biodegradable CILs.
-
Methods for treating an inflammatory condition or inhibiting JNK申请人:——公开号:US20040127536A1公开(公告)日:2004-07-01This invention is generally directed to Indazole Derivatives having the following structure: 1 or pharmaceutically acceptable salt thereof, wherein R 1 , R 2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of diseases and disorders that are responsive to JNK inhibition, such as an inflammatory disease or disorder. Thus, methods of treating such diseases and disorders are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
-
Indazole compounds, compositions thereof and methods of treatment therewith申请人:Bhagwat S. Shripad公开号:US20050009876A1公开(公告)日:2005-01-13This invention is generally directed to the use of Indazole Compounds for treating or preventing diseases associated with protein kinases, including tyrosine kinases, such as proliferative diseases, inflammatory diseases, abnormal angiogenesis and diseases related thereto, atherosclerosis, macular degeneration, diabetes, obesity, pain and others. The methods comprise the administration to a patient in need thereof of an effective amount of an indazole compound that inhibits, modulates or regulates tyrosine kinase signal transduction. Novel indazole compounds or pharmaceutically acceptable salt thereof are presented herein.
-
Characterization of FabG and FabI of the<i>Streptomyces coelicolor</i>Dissociated Fatty Acid Synthase作者:Renu Singh、Kevin A. ReynoldsDOI:10.1002/cbic.201402670日期:2015.3.2Functional crosstalk: β‐Ketoacyl‐acyl carrier protein (ACP; FabG) and enoyl‐ACP reductase (FabI) from Streptomyces coelicolor were identified and characterized. Kinetic analysis demonstrated that these enzymes process straight and branched‐chain substrates along with the ACPs from both fatty acid and undecylprodiginine biosynthetic pathways. This relaxed substrate specificity allow these enzymes to
表征谱图
-
氢谱1HNMR
-
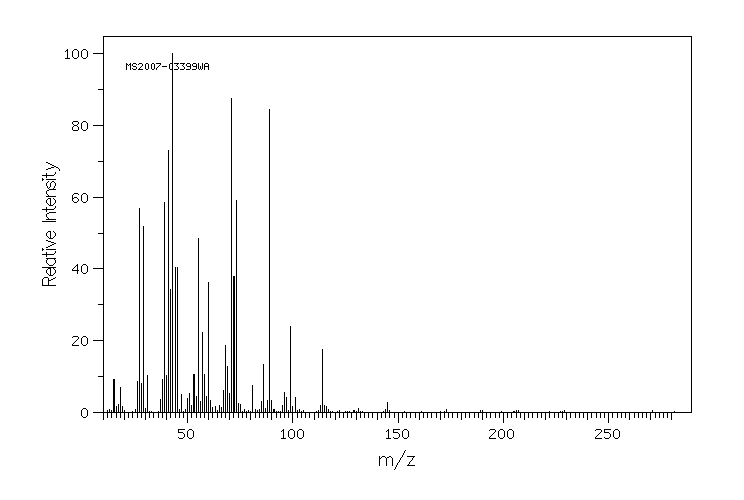
质谱MS
-
碳谱13CNMR
-
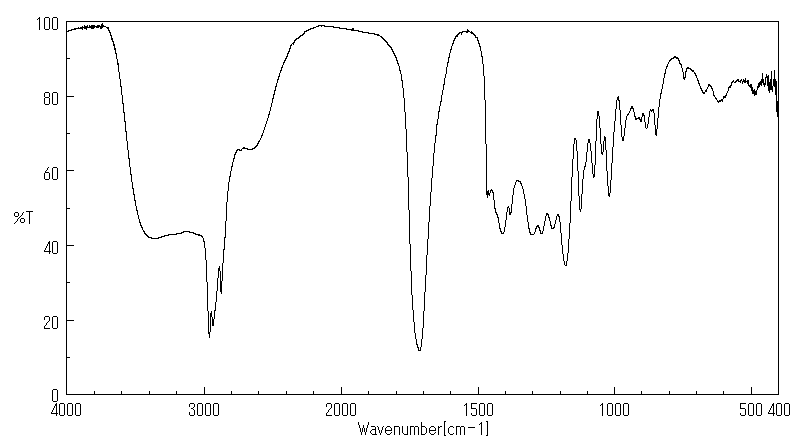
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2-苯基-3-羟基丙酸
(2S,3R)-2,3-二羟基-3-(2-吡啶基)丙酸乙酯,N-氧化物
麦拉乳酸
阿拉伯碳酸氢二钾
铵;铈(+3)阳离子;(2R,3R)-2,3-二羟基丁烷二酸盐
钡二{8-[3-(2-羟基辛基)-2-环氧乙烷基]辛酸酯}
钠3-脱氧-D-阿拉伯糖-己酮酸酯
钠3-脱氧-D-木糖基-己酮酸酯
钠(3R,5R)-3,5-二羟基-7-[(1S,2S,6R,8S,8aR)-8-羟基-2,6-二甲基-1,2,6,7,8,8A-六氢-1-萘基]庚酸酯
钠(2S)-2-羟基(13C3)丙酸酯
酮酯
酒石酸锂单水合物
酒石酸铬
酒石酸铜(II)一水
酒石酸钾锑
酒石酸钾
酒石酸钠
酒石酸鐵(III)鉀
酒石酸辛酯钠盐
酒石酸羟吡啶
酒石酸氢钾
酒石酸异丙酯
酒石酸二磺基琥珀酰亚胺酯
酒石酸二琥珀酰亚胺酯
酒石酸二戊酯
酒石酸二仲丁酯
酒石酸二丙酯
辛酸,8-氯-6-羟基-,(6R)-
辛伐他汀钾盐
辛伐他汀钠盐
辛伐他汀酸
超支化BIS-MPA聚酯-64-羟基,4代
西托溴铵
表洛伐他汀羟基酸钠盐
葡萄糖酸镍
葡萄糖酸锶
葡萄糖酸锰
葡萄糖酸汞
葡萄糖酸亚铁
莫那可林J酸
苹果酸镁
苹果酸镁
苹果酸铵盐
苹果酸钙
苹果酸氢钠
苹果酸氢钠
苹果酸根
苹果酸二烯丙酯
苹果酸二乙基己酯
苹果酸乙酯(S)-2-羟基丁二酸1-乙酯(苹果酸杂质S)