4-苄氧基-1-硝基苯 | 1145-76-2
中文名称
4-苄氧基-1-硝基苯
中文别名
1-苯甲氧基-4-硝基苯;苯甲基4-硝基苯醚
英文名称
benzyl 4-nitrophenyl ether
英文别名
1-(benzyloxy)-4-nitrobenzene;Benzene, 1-nitro-4-(phenylmethoxy)-;1-nitro-4-phenylmethoxybenzene
CAS
1145-76-2
化学式
C13H11NO3
mdl
MFCD00024672
分子量
229.235
InChiKey
YOVUXLHIVNBVKO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:105.0 to 109.0 °C
-
沸点:386.2±17.0 °C(Predicted)
-
密度:1.232±0.06 g/cm3(Predicted)
-
最大波长(λmax):294nm(Hexane)(lit.)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.076
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
海关编码:2909309090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温且干燥
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Benzyl 4-nitrophenyl ether
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Skin irritation (Category 2)
Serious eye damage (Category 1)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Irritating to respiratory system and skin. Risk of serious damage to eyes.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Danger
Hazard statement(s)
H315 Causes skin irritation.
H318 Causes serious eye damage.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P280 Wear protective gloves/ eye protection/ face protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R37/38 Irritating to respiratory system and skin.
R41 Risk of serious damage to eyes.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S39 Wear eye/face protection.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C13H11NO3
Molecular Weight : 229,23 g/mol
Component Concentration
Benzyl 4-nitrophenyl ether
-
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N100 (US) or type P3 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 2,743
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
Prolonged or repeated exposure may cause allergic reactions in certain sensitive individuals. The
preceding data, or interpretation of data, was determined using Quantitative Structure Activity Relationship
(QSAR) modeling.
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. Causes skin irritation.
Eyes Causes eye burns.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-硝基苯甲醚 4-Nitroanisole 100-17-4 C7H7NO3 153.137 4-硝基苯乙醚 1-ethoxy-4-nitrobenzene 100-29-8 C8H9NO3 167.164 —— (benzyloxy)benzene 31324-44-4 C13H12O 184.238 4-硝基苯基碳酸苄酯 benzyl 4-nitrophenyl carbonate 13795-24-9 C14H11NO5 273.245 对硝基苯酚 4-nitro-phenol 100-02-7 C6H5NO3 139.111 1-苄氧基-4-碘苯 1-benzyloxy-4-iodobenzene 19578-68-8 C13H11IO 310.134 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4-nitro-benzyl)-(4-nitro-phenyl)-ether 67565-49-5 C13H10N2O5 274.233 —— N-(4-(benzyloxy)phenyl)hydroxylamine —— C13H13NO2 215.252 4-苄氧基苯胺 p-benzyloxyaniline 6373-46-2 C13H13NO 199.252 4-苄氧基苯异氰酸酯 4-benzyloxy-phenyl isocyanate 50528-73-9 C14H11NO2 225.247 4-(苄氧基)苯基异硫氰酸酯 4-benzyloxyphenyl isothiocyanate 139768-71-1 C14H11NOS 241.313 —— 4,4-bis(phenylmethoxy)azoxybenzene 101653-61-6 C26H22N2O3 410.472 4-硝基苯基乙酸酯 4-nitrophenol acetate 830-03-5 C8H7NO4 181.148 —— 4-benzoyloxy-aniline 720-98-9 C13H11NO2 213.236 对硝基苯酚 4-nitro-phenol 100-02-7 C6H5NO3 139.111 —— 5-benzyloxy-2-nitrophenylacetonitrile 15566-30-0 C15H12N2O3 268.272 —— N-benzyl-4-(benzyloxy)aniline 39860-72-5 C20H19NO 289.377 —— (2,4-dinitro-phenyl)-(4-nitro-benzyl)-ether 4279-47-4 C13H9N3O7 319.23 —— N-(4-benzyloxyphenyl)-2-bromoacetamide 349120-98-5 C15H14BrNO2 320.186 N-(4-苄氧基-苯基)-2-氯-乙酰胺 N-(4-(benzyloxy)phenyl)-2-chloroacetamide 19514-92-2 C15H14ClNO2 275.735 —— (E)-2-(((4-(benzyloxy)phenyl)imino)methyl)-4-nitrophenol —— C20H16N2O4 348.358 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:四氢咔唑衍生物作为潜在降糖药的设计、合成和评价摘要:基于 ZG02 设计和合成了两个系列的四氢咔唑衍生物,ZG02 是我们之前研究中开发的有前景的候选物。在 HepG2 细胞系中筛选新制备的化合物的葡萄糖消耗活性。氮杂-四氢咔唑化合物12b显示出最有效的降血糖活性,与溶剂对照相比,葡萄糖消耗增加了 45%,其活性比阳性对照化合物(二甲双胍和 ZG02)高约 1.2 倍。对潜在机制的研究表明,12b可能通过激活 AMPK 途径表现出降血糖活性。代谢稳定性分析表明,12b在来自 SD 大鼠的人造胃肠液和血浆中均显示出良好的稳定性。进行了口服葡萄糖耐量试验 (OGTT),结果进一步证实12b是一种有效的降血糖药。DOI:10.1016/j.bioorg.2021.105172
-
作为产物:描述:4-硝基苯基碳酸苄酯 在 allyl(cyclopentadiene)palladium(II) 、 双(2-二苯基磷苯基)醚 作用下, 以 甲苯 为溶剂, 反应 22.08h, 以89%的产率得到4-苄氧基-1-硝基苯参考文献:名称:在中性条件下苯酚的苄基保护:钯催化的苯酚苄基化。摘要:通过使用Pd(eta3-C3H5)Cp-DPEphos催化剂在中性条件下实现苯酚的苄基保护。钯催化剂通过脱羧醚化将芳基碳酸苄酯有效地转化为苄基保护的酚。或者,在催化剂的存在下用苯酚进行碳酸苄基甲基酯的亲核取代,得到芳基苄基醚。DOI:10.1021/ol800548t
文献信息
-
Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium作者:F.D. Bellamy、K. OuDOI:10.1016/s0040-4039(01)80041-1日期:1984.1Aromatic nitro compounds are readily reduced by SnCl2, 2 H2O in alcohol or ethyl acetate or by anhydrous SnCl2 in alcohol where other reducible or acid sensitive groups such as aldehyde, ketone, ester, cyano, halogen and O-benzyl remain unaffected.
-
Reduction of Nitroarenes to Anilines with a Benzothiazoline: Application to Enantioselective Synthesis of 2-Arylquinoline Derivatives作者:Masamichi Miyagawa、Ryota Yamamoto、Nanako Kobayashi、Takahiko AkiyamaDOI:10.1055/s-0037-1611639日期:2019.3The metal-free reduction of nitroarenes to aniline derivatives was accomplished in a short time by using a benzothiazoline as the hydrogen donor in combination with a Bronsted acid. An enantioselective synthesis of 2-arylquinolines was achieved by using 1-aryl-3-(2-nitrophenyl)propan-1-ones as starting materials and a combination of a benzothiazoline and a chiral phosphoric acid.
-
Stabilisation of gold nanoparticles by N-heterocyclic thiones作者:Leonardo C. Moraes、Bertrand Lacroix、Rute C. Figueiredo、Patricia Lara、Javier Rojo、Salvador ConejeroDOI:10.1039/c7dt01856h日期:——Gold nanoparticles (Au-NPs) have been prepared using N-heterocyclic thiones (NHTs) as ligand stabilisers. These Au-NPs have been shown to be very stable, even in air, and have been characterized by a combination of several techniques (TEM, HR-TEM, STEM-HAADF, EDX, DLS, elemental analysis and 1H NMR). These nanoparticles are active in the catalytic reduction of nitroarenes to anilines.
-
Ambident Reactivity of Phenolate Anions Revisited: A Quantitative Approach to Phenolate Reactivities作者:Robert J. Mayer、Martin Breugst、Nathalie Hampel、Armin R. Ofial、Herbert MayrDOI:10.1021/acs.joc.9b01485日期:2019.7.19reactions (C versus O attack) are opposite to the predictions by the principle of hard and soft acids and bases, we performed a comprehensive experimental and computational investigation of phenolate reactivities. Rate and equilibrium constants for the reactions of various phenolate ions with benzhydrylium ions (Aryl2CH+) and structurally related quinone methides have been determined photometrically in polar根据观察结果,酚酸反应(C与O攻击)的区域选择性与硬酸和软酸和碱的原理预测相反,我们对酚酸反应性进行了全面的实验和计算研究。各种酚酸根离子与二苯甲基铵离子(Aryl 2 CH +)和结构相关的醌甲基化物已在极性非质子传递溶剂中通过光度法测定。在SMD(MeCN)/ M06-2X / 6-31 + G(d,p)级别上进行的量子化学计算证实,在动力学控制的条件下,通常有利于O攻击,而在热力学控制的条件下,则有利于C攻击。例外是与强亲电试剂的扩散受限反应,该反应会产生由O和C攻击产生的产物混合物,以及在非极性溶剂中与金属醇盐的反应,其中强离子对阻止了氧气的攻击。刘易斯碱度(LB)和亲核性(N,s N)在这项工作中确定的酚盐参数可以用来预测它们与亲电试剂的反应是动力学还是热力学控制,以及速率是受活化限制还是受扩散限制。酚盐与碳正离子与吉布斯能量进行单电子转移反应所测得的速率常数的比较表明,这些反应是通过极性机理进行的。
-
Zinc phthalocyanine with PEG-400 as a recyclable catalytic system for selective reduction of aromatic nitro compounds作者:Upendra Sharma、Neeraj Kumar、Praveen Kumar Verma、Vishal Kumar、Bikram SinghDOI:10.1039/c2gc35452g日期:——for the first time. The present catalytic system was successfully employed for the reduction of carbonyl and ester compounds to corresponding alcohols and reductive amination of benzaldehydes with primary amines to form corresponding secondary amines. Remarkable advantages of the present catalytic method include low loading of metal, avoidance of toxic ligands and high isolated yields. The catalyst was
表征谱图
-
氢谱1HNMR
-
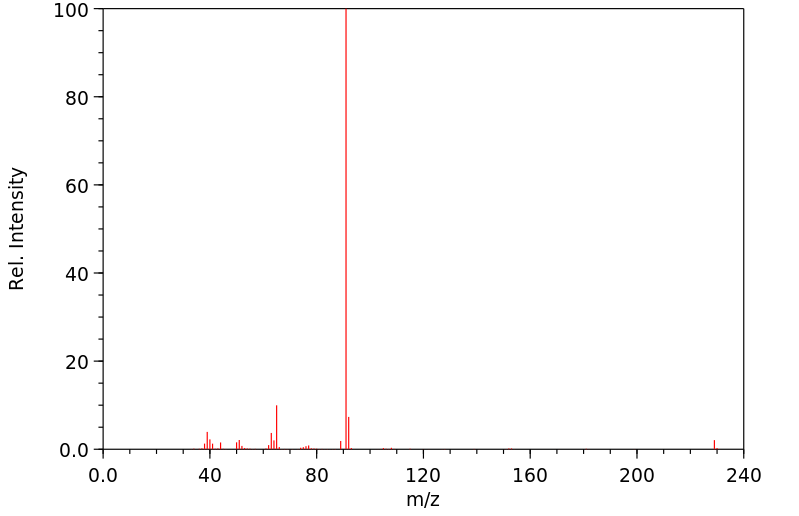
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫