tris(dipivaloylmethanato)europium(III) | 15522-71-1
物质功能分类
中文名称
——
中文别名
——
英文名称
tris(dipivaloylmethanato)europium(III)
英文别名
Eu(2,2,6,6-tetramethyl-3,5-heptanedionato)3;Eu(2,2,6,6-tetramethyl-3,5-heptanedionate)3;Eu(dpm)3;europium tris(2,2,6,6-tetramethyl-3,5-heptanedionato);europium tris(2,2,6,6-tetramethyl-3,5-heptanedionate);europium(III) tris(2,2,6,6-tetramethyl-3,5-heptanedionate);tris(2,2,6,6-tetramethyl-3,5-heptanedionato) europium(III);tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium;tris(dipivaloylmethanato)Eu(III);europium dipivaloylmethanate;Eu(2,2,4,4-tetramethyl-3,5-heptanedionate)3;[Eu(2,2,6,6-tetramethyl-3,5-heptadioate)3];[Eu(dipivaloylmethane)3];[Eu(thd)3];Europium(III) 2,2,6,6-tetramethylheptane-3,5-dione;europium(3+);(Z)-2,2,6,6-tetramethyl-5-oxohept-3-en-3-olate
CAS
15522-71-1
化学式
C33H57EuO6
mdl
——
分子量
701.772
InChiKey
DVUACRCEVJAWMS-LWTKGLMZSA-K
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.68
-
重原子数:40
-
可旋转键数:9
-
环数:0.0
-
sp3杂化的碳原子比例:0.73
-
拓扑面积:120
-
氢给体数:0
-
氢受体数:6
安全信息
-
TSCA:Yes
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914509090
-
储存条件:常温密闭避光,存于通风干燥处。
SDS
Tris(2,2,6,6-tetramethyl-3,5- Revision number: 5
heptanedionato)europium(III) [NMR Shift Reagent]
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium(III) [NMR Shift Reagent]
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
Signal word No signal word
Hazard statements None
Precautionary statements: None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
Components: Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium(III) [NMR Shift Reagent]
....
Percent:
CAS Number: 15522-71-1
Eu(dpm)3 , Europium(III) Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)
Synonyms:
Chemical Formula: C33H57EuO6
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Tris(2,2,6,6-tetramethyl-3,5-
heptanedionato)europium(III) [NMR Shift
Reagent]
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: Pale yellow - Yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:190°C
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
Tris(2,2,6,6-tetramethyl-3,5-
heptanedionato)europium(III) [NMR Shift
Reagent]
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Tris(2,2,6,6-tetramethyl-3,5-
heptanedionato)europium(III) [NMR Shift
Reagent]
SECTION 16 - ADDITIONAL INFORMATION
N/A
heptanedionato)europium(III) [NMR Shift Reagent]
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium(III) [NMR Shift Reagent]
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
Signal word No signal word
Hazard statements None
Precautionary statements: None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
Components: Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium(III) [NMR Shift Reagent]
....
Percent:
CAS Number: 15522-71-1
Eu(dpm)3 , Europium(III) Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)
Synonyms:
Chemical Formula: C33H57EuO6
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Tris(2,2,6,6-tetramethyl-3,5-
heptanedionato)europium(III) [NMR Shift
Reagent]
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: Pale yellow - Yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:190°C
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
Tris(2,2,6,6-tetramethyl-3,5-
heptanedionato)europium(III) [NMR Shift
Reagent]
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Tris(2,2,6,6-tetramethyl-3,5-
heptanedionato)europium(III) [NMR Shift
Reagent]
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:tris(dipivaloylmethanato)europium(III) 以 neat (no solvent) 为溶剂, 生成 氢化铕参考文献:名称:Temperature dependent rate constants for the reactions of gas phase lanthanides with N2O摘要:The reactivity of gas phase lanthanide (Ln) atoms (Ln=La–Yb with the exception of Pm) with N2O from 298 to 623 K is reported. Lanthanide atoms were produced by the photodissociation of Ln(TMHD)3 (TMHD=2,2,6,6-tetramethyl-3,5-heptanato ion) and detected by laser-induced fluorescence. Large variations in the reaction rate constants are observed. The bimolecular rate constants are described in Arrhenius form by k[Ce(1G4)]=(1.9±0.5)×10−10 exp(−0.8±0.8 kJ mol−1/RT); Pr(4I9/2), (3.6±1.2)×10−10 exp(−8.0±1.2 kJ mol−1/RT); Nd(5I4), (3.4±0.4)×10−10 exp(−8.8±0.5 kJ mol−1/RT); Sm(7F0), (3.2±1.1)×10−10 exp(−11.2±1.2 kJ mol−1/RT); Eu(8S7/2), (2.7±0.4)±10−10 exp(−12.7±0.5 kJ mol−1/RT); Gd(9D2), (2.0±0.3)×10−10 exp(−6.4±0.5 kJ mol−1/RT); Tb(6H15/2), (2.9±0.5)×10−10 exp(−10.9±0.6 kJ mol−1/RT); Dy(5I8), (3.4±0.8)×10−10 exp(−16.2±0.8 kJ mol−1/RT); Ho(4I15/2), (2.9±0.5)×10−10 exp(−17.1±0.6 kJ mol−1/RT); Er(3H6), (3.3±1.2)×10−10 exp(−18.4±1.2 kJ mol−1/RT); Tm(2F7/2), (3.5±0.6)×10−10 exp(−19.5±0.6 kJ mol−1/RT); Yb(1S0), (2.5±0.2)×10−10 exp(−20.2±0.3 kJ mol−1/RT) where the uncertainties represent ±2σ. The reaction barriers are found to correlate to the energy required to promote an electron out of the 6s subshell.DOI:10.1063/1.479336
-
作为产物:描述:[tris(dipivaloylmethanato)diaqua]Eu(III) 以 neat (no solvent) 为溶剂, 生成 tris(dipivaloylmethanato)europium(III)参考文献:名称:辅助配体N,N-二甲基氨基乙醇在二聚体β-二酮酸酯中Eu(III)和Tb(III)发光敏化中的作用。摘要:两种二聚体复合物[Ln2(hfa)6(mu2-O(CH2)2NHMe2)2]和[Ln(thd)2(mu2,eta2-O(CH2)2NMe2)] 2(Ln = YIII,EuIII,GdIII ,TbIII,TmIII,LuIII; hfa- =六氟乙酰丙酮,thd- =二新戊酰基甲酰氨基)分别通过[Ln(hfa)3(H2O)2]和[Ln(thd)3]与N,N-二甲基氨基乙醇的反应制得并具有充分的特征。对TbIII化合物进行的X射线单晶分析证实了其二聚体结构。N,N-二甲基氨基乙醇的配位方式取决于β-二酮酸酯的性质。在[Tb2(hfa)6(mu2-O(CH2)2NHMe2)2]中,八配位的TbIII离子采用扭曲的方形反棱柱配位环境,并被两个带有Tb1的两性离子N,N-二甲基氨基乙醇配体O桥。 Tb2分离为3.684(1)A。在[Tb(thd)2(mu2,eta2-O(CH2)2NMe2)] 2中,NDOI:10.1021/jp711305u
-
作为试剂:描述:苯甲酰溴 、 氧化环己烯 在 tris(dipivaloylmethanato)europium(III) 作用下, 以 苯 为溶剂, 反应 15.0h, 以91%的产率得到2-bromocyclohexyl benzoate参考文献:名称:镧系元素催化环氧化物与酰基卤的开环反应摘要:Eu(dpm)3 [dpm:二新戊酰基甲酸酯]催化环氧化物与酰基卤的开环反应,得到相应的2-卤代烷基酯。在环己烯氧化物反应的情况下,立体化学过程被确认为反式加成。DOI:10.1016/s0040-4039(98)00831-4
文献信息
-
Crystal Structures of [Dy(dpm)3]2 and Dy(dpm)3, Luminescent and X-Ray Fluorescent Study of Lanthanide(III) Tris-Dipivaloylmethanates作者:Huang Shen、A. S. Berezin、O. V. Antonova、V. V. Zvereva、I. V. Korolkov、N. V. Pervukhina、S. A. Prokhorova、P. A. StabnikovDOI:10.1134/s0022476618030265日期:2018.5Crystal structures of [Dy(dpm)3]2 (1) at 155(2) K (space group P21/n, a = 12.2191(3) Å, b = 27.6044(6) Å, с = 21.8615(5) Å, β = 105.172(1)°, V = 7116.9(3) Å3, Z = 4) and Dy(dpm)3 (2) at 200(2) K (space group Pmn21, a = 17.8741(7) Å, b = 10.5639(4) Å, с = 9.8336(4) Å, V = 1856.78(13) Å3, Z = 2) are determined. The structure of complex 1 is similar to the previously known dimeric packings [Ln(dpm)3]2[Dy(dpm)3]2 (1) 在 155(2) K 的晶体结构(空间群 P21/n,a = 12.2191(3) Å,b = 27.6044(6) Å,с = 21.8615(5) Å , β = 105.172(1)°, V = 7116.9(3) Å3, Z = 4) 和 Dy(dpm)3 (2) 在 200(2) K(空间群 Pmn21, a = 17.8741(7) Å, b = 10.5639(4) Å, ñ = 9.8336(4) Å, V = 1856.78(13) Å3, Z = 2)。配合物 1 的结构类似于先前已知的二聚体填料 [Ln(dpm)3]2 (Ln = La, Pr, Nd, Eu, Gd, Tb)。配合物 2 与先前研究的分子配合物 Er(dpm)3 和 Lu(dpm)3 是同构的。在室温下,发现 Tb(dpm)3 的发光量子产率 (QY) 达到 77%,Dy(dpm)3
-
The stoicheiometry and stability constants of adducts of lanthanide shift reagents作者:Graham A. Catton、F. Alan Hart、Gerard P. MossDOI:10.1039/dt9760000208日期:——Application of Job's method to the visible spectra of adducts of tris(2,2,6,6-tetramethylheptane-3,5-dionato)-holmium in carbon tetrachloride shows that 1:1 adducts are formed with stability constants which vary considerably with the nature of the added ligand.
-
Mechanically induced chemiluminescence in the dispiro(adamantane-1,2-dioxetane)?Eu(fod)3 system作者:V. A. Antipin、A. I. Voloshin、S. S. Ostakhov、Y. P. KazakovDOI:10.1007/bf01431597日期:1996.5Luminescence accompanying an impact mechanical treatment of solid particles of the complex of Eu(fod)3 (fod is 1,1,1,2,2,3,3-heptafluoro-7,7-dimethyloctane-4,6-dione) with dispiro(adamantane-1,2-dioxetane) (1) was discovered and studied. The luminescence has a complex structure, and its spectrum belongs to the excited EuIII ions. The emission of light is observed only in a mechanical mixture of the
-
Studies on the mixed ligand complexes of rare earths with dipivaloylmethane and pyrazine作者:M. Shameem Ansari、Naseer AhmadDOI:10.1016/0022-1902(75)80838-4日期:1975.10The mixed ligand complexes, tris(dipivaloylmethanato) pyrazinates of trivalent lanthanide (except Ce, Pm and Lu) and yttrium ions were synthesised and characterised by elemental analyses, melting points, thermogravimetric analyses, magnetic moments, molar conductances and i.r. spectrum. The complexes are Ln(dpm)3Pz where Ln = trivalent lanthanide or yttrium ion, dpm = dipivaloylmethane and Pz = pyrazine
-
Preparation and X-ray crystal structure (for Ln = Sm) of (µ-phthalocyaninato)bis[di(2,2,6,6-tetramethylheptane-3,5-dionato)Ln<sup>III</sup>](Ln = Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Y)作者:Hiroshi Sugimoto、Teruaki Higashi、Atsushi Maeda、Masayasu Mori、Hideki Masuda、Tooru TagaDOI:10.1039/c39830001234日期:——The reaction of dilithium phthalocyanine with tris(2,2,6,6-tetramethylheptane-3,5-dionato)LnIII(Ln = Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Y) affords a series of (µ-phthalocyaninato)(diketonato)4Ln2complexes; the X-ray crystal structure of the samarium compound shows that the phthalocyanine ligand bridges two samarium atoms each co-ordinated by two β-diketonato ligands, and important molecular parameters
表征谱图
-
氢谱1HNMR
-
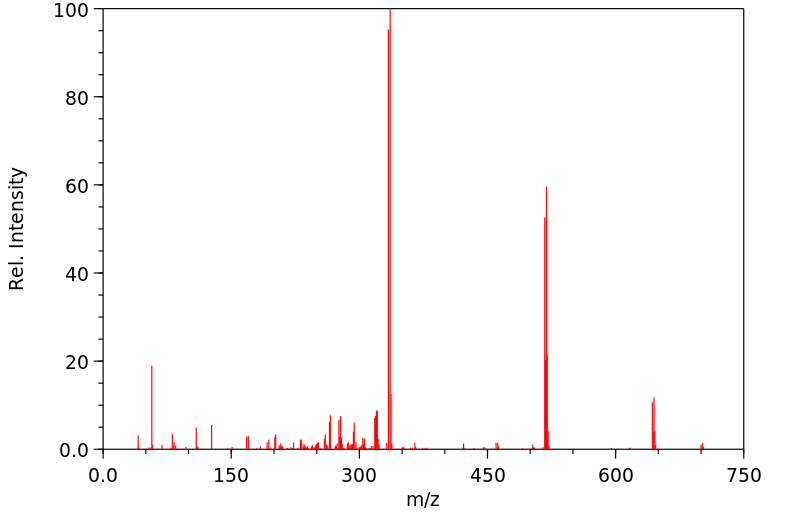
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷