苯 | 71-43-2
中文名称
苯
中文别名
1,3,5-环己三烯;环己-1,3,5-三烯;安息油;苯查儿;纯苯;精苯;净苯;困净苯;溶剂苯
英文名称
benzene
英文别名
——
CAS
71-43-2
化学式
C6H6
mdl
MFCD00003009
分子量
78.1136
InChiKey
UHOVQNZJYSORNB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:5.5 °C (lit.)
-
沸点:80 °C (lit.)
-
密度:0.874 g/mL at 25 °C (lit.)
-
蒸气密度:2.77 (vs air)
-
闪点:12 °F
-
溶解度:与乙醇、氯仿、二氯甲烷、乙醚、丙酮和乙酸混溶。
-
最大波长(λmax):λ: 280 nm Amax: 1.0λ: 290 nm Amax: 0.15λ: 300 nm Amax: 0.06λ: 330 nm Amax: 0.02λ: 350-400 nm Amax: 0.01
-
介电常数:2.3(20℃)
-
暴露限值:TLV-TWA 10 ppm (~32 mg/m3) (ACGIH and OSHA); ceiling 25 ppm (~80 mg/m3) (OSHA and MSHA); peak 50 ppm (~160 mg/m3)/10 min/8 h (OSHA); carcinogenicity: Suspected Human Carcinogen (ACGIH), Human Sufficient Evidence (IARC).
-
LogP:2.130
-
物理描述:Clear, colorless to light yellow liquid at room temperature. Benzene is a solid below 42°F (5.6°C).
-
颜色/状态:Clear, colorless liquid
-
气味:Aromatic odor
-
味道:Taste threshold in water is 0.5-4.5 mg/l.
-
蒸汽密度:2.77 (NTP, 1992) (Relative to Air)
-
蒸汽压力:94.8 mm Hg at 25 °C
-
亨利常数:0.01 atm-m3/mole
-
大气OH速率常数:1.23e-12 cm3/molecule*sec
-
稳定性/保质期:
-
苯的主要化学反应包括加成、取代和开环反应。在浓硫酸或硝酸的作用下,苯容易发生取代反应生成硝基苯;与浓硫酸或发烟硫酸反应可生成苯磺酸;以三氯化铁等金属卤化物为催化剂,在较低温度下进行卤化反应可生成卤代苯;以三氯化铝为催化剂,苯可以与烯烃、卤代烃发生烷基化反应生成烷基苯,也可以与酸酐、酰氯发生酰化反应生成酰基苯。在氧化钒催化剂的作用下,苯可通过氧或空气的氧化生成顺丁烯二酸酐;加热到700℃时,苯会发生裂解生成碳、氢及少量甲烷和乙烯等产物;使用铂或镍作催化剂进行加氢反应可生成环己烷;以氯化锌为催化剂与甲醛和氯化氢发生氯甲基化反应可生成苄基氯。尽管苯的环结构比较稳定,但仍然不与硝酸、高锰酸钾、重铬酸盐等氧化剂发生反应。
-
苯具有高折射性和强烈的芳香味,易燃且有毒。它能溶于乙醇、乙醚、丙酮、四氯化碳、二硫化碳和醋酸中,并微溶于水;对金属无腐蚀性,但低品级的苯含有硫杂质可能会对铜及其他金属产生腐蚀作用。液体苯具有脱脂作用,可通过皮肤被吸收而中毒,因此应避免直接接触。
-
苯蒸气与空气可形成爆炸性混合物,其爆炸极限为1.5%-8%(体积)。
-
苯是稳定的。
-
苯的禁配物质包括强氧化剂、酸类和卤素等。
-
苯不会发生聚合反应。
-
自燃温度:928 °F (498 °C)
-
粘度:0.604 mPa.s at 25 °C
-
燃烧热:-3267.6 kJ/mol (liquid)
-
汽化热:33.83 kJ/mol at 25 °C
-
表面张力:28.22 mN/m at 25 °C
-
电离电位:9.24 eV
-
气味阈值:4.68 PPM
-
折光率:Index of refraction: 1.5011 at 20 °C/D
-
相对蒸发率:2.8 (Ether = 1)
-
保留指数:647.5;638.32;638.98;645;630;640.2;639;638.6;640.7;645;645;646;643.1;640.5;641.77;641.83;630;644;654;641.72;642;642;654;654;648;648.9;651.4;640.61;643.07;644.46;654;642.7;645.26;646;647.11;642;650;648;647;645;646.8;648.9;650.2;651.1;652.6;657.6;638;649.89;651.77;653.93;656.09;658.35;660.36;663.6;664.4;666.96;670.44;672.02;674.86;672;684.6;629;670.45;654.52;662;663;663;668;654;649.7;644;677;654.7;660.2;663.2;667.4;649.4;656.3;643;678.1;688;653.5;653.8;654.2;654.2;654.4;649;647.2;669.9;666;655;655;664;664;663.1;670.7;663;651.3;651.4;655.4;655.8;654;655;661.6;649;653;658;661;662;664;672;652.1;654.1;656;658.3;681;655;681;668;675;669;662;662;664;664;647;649;651;653;656;658;653.8;654.4;657.1;664;652;659;670;664;671;678;642;650;651;653;654;645.7;648;648.4;650.3;650.5;652.6;652.7;654.9;655.2;657.2;658.2;659.7;663.6;670.7;677.8;646;659.1;647;648;674;650;654.7;654.7;654.8;654.8;654.8;654.8;652;654;654;656;656;692;660;648;643;653;657;660;663;667;668;668;637;658;662;668;668;668;668;672;678;670;657;651;654.1;656;644.9;647.7;647.5;643.1;656.1;644.1;639.25;645.5;654;664;652;651.5;638.83;649;640;651.1;649;654;657;655;648;654;657;664;657;643;640;653;651;640;658;657;663;664;670;670;649;638;640;640;660;665;654;655;655;656;657;658;659;653;644;647;648.6;658;663.5;664.6;642;644;643;644;638;634;644;640.5;665;644;641;653;669;673;651;655;660;642;641;644;670;648;652;653;665;665;654
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
The major metabolitesof benzene metabolism are phenol, hydroquinone, and catechol. These metabolites are interactive and can affect the rate of each other's metabolism because they are substrates for the P-450 enzyme system. The route of exposure has little effect on the subsequent metabolism of benzene to hemotoxic metabolites.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Metabolic products in rat ... are phenol, hydroquinone, catechol, hydroxyhydroquinone, & phenylmercapturic acid. Conjugated phenols have been reported ... except for a small amt of free phenol, all the phenolic metabolites were excreted in conjugated form. When (3)H-benzene was admin to mice, (3)H2O was also recovered from urine.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Yields N-acetyl-S-phenyl-cysteine in rat. Yields benzyl alcohol in guinea pigs. ... Yields cis-1,2-dihydro-1,2-dihydroxybenzene in pseudomonas. Phenol in pseudomonas & achromobacter. Yields cis,cis-muconic acid in rabbit. /From table/
来源:Hazardous Substances Data Bank (HSDB)
代谢
In the rabbit, the major hydroxylation product of benzene was phenol, which along with some catechol and hydroquinone, was found in the urine conjugated with ethereal sulfate or glucuronic acid.
来源:Hazardous Substances Data Bank (HSDB)
代谢
苯已知的人类代谢物包括酚。
Benzene has known human metabolites that include phenol.
来源:NORMAN Suspect List Exchange
毒理性
识别和使用:苯是一种无色透明的液体,具有甜美的芳香气味。它主要用于制造其他化学品,包括洗涤剂、杀虫剂、塑料和树脂、合成橡胶、航空燃料、药品、染料、炸药、PCB汽油、香料和香水、油漆和涂料、尼龙中间体、摄影化学品。人类暴露和毒性:苯暴露的即时征兆和症状:吸入高浓度苯的人可能会出现嗜睡、眩晕、心跳过快或不规则、头痛、震颤、混乱、昏迷、死亡。食用含有高浓度苯的食物或饮料会导致呕吐、胃部刺激、眩晕、嗜睡、抽搐、心跳过快或不规则、死亡。长期(一年或更长时间)暴露于苯会对骨髓产生有害影响,导致贫血和过度出血。它还可能影响免疫系统,增加感染的机会。一些女性在吸入高浓度苯数月后,月经周期不规律,卵巢体积减小。高浓度苯暴露导致的急性死亡是由于劳累和肾上腺素释放引起的室性纤维颤动。苯在人类中可引起癌症。在中国12个城市的233家苯工厂和83家对照工厂进行了一项回顾性队列研究。苯队列和控制队列分别包括28,460名暴露于苯的工人和28,257名对照工人。苯队列的白血病死亡率为每10万人年14例,对照组为每10万人年2例。大多数(76.6%)苯白血病为急性型。苯白血病的死亡率在有机合成厂最高,其次是油漆和橡胶合成行业。患有白血病的患者接触的苯浓度范围为10至1000毫克/立方米(大部分为50至500毫克/立方米)。苯在人类中具有基因毒性:据报道,暴露工人的培养淋巴细胞中染色单体和等色单体断裂的频率显著增加,外周血淋巴细胞染色体畸变的显著增加。大鼠肝脏微粒体对苯的代谢激活诱导了培养的人类淋巴细胞的姐妹染色单体交换和细胞分裂延迟。职业暴露于苯可能通过吸入和皮肤接触发生。一般人群可能通过吸入环境空气、食物和饮用水以及接触含有苯的消费品而暴露于苯。动物毒性研究:实验动物研究,包括吸入和口服,也支持暴露于苯会增加多个器官系统癌症风险的证据,包括造血系统、口腔和鼻腔、肝脏、前胃、包皮腺、肺、卵巢和乳腺。在封闭室中暴露于3,526-8,224 ppm苯的大鼠15分钟内,室性异位搏动数量增加。在发育研究中,大鼠每天接触10、50或500 ppm(32、160和1600毫克/立方米)的苯7小时,脑部和骨骼缺陷的发生率较低。大鼠在交配前连续10天暴露于209.7 ppm,完全未怀孕。接触19.8 ppm的1/10大鼠吸收了胚胎。基因毒性研究显示,用单次或每日多次剂量的苯处理小鼠、大鼠和兔子的骨髓细胞中诱导了染色体畸变,剂量范围约为0.2至2.0毫升/千克,通过皮下或腹腔注射给药。苯的主要代谢物是酚、氢醌和对苯二酚。暴露途径对苯代谢为血液毒物代谢物的影响很小。生态毒性研究:在8°C的人工海水中,50 ppm的幼鲑鱼24小时内的死亡率为12/20,96小时内的死亡率为30/30。鲱鱼和沙丁鱼幼虫研究表明,35-45 ppm的苯会延迟卵的发育并产生异常幼虫;10-35 ppm的苯会延迟幼虫的发育、减少摄食和生长,并增加呼吸。在静态系统中暴露于亚致死浓度苯(0.1或5.0 ppm)的蓝蟹幼蟹完成蜕皮周期所需的时间增加(苯暴露的蟹需要50天,而对照组为33天),再生肢芽的生长速度减慢,线粒体ATP酶的活性降低。蟹暴露于1.0 ppm苯时,耗氧量减少。
IDENTIFICATION AND USE: Benzene is a clear, colorless liquid with a sweet aromatic odor. It is used mainly as a starting material in manufacturing other chemicals, including detergents, pesticides, plastics and resins, synthetic rubber, aviation fuel, pharmaceuticals, dye, explosives, PCB gasoline, flavors and perfumes, paints and coatings, nylon intermediates, photographic chemicals. HUMAN EXPOSURE AND TOXICITY: Immediate signs and symptoms of exposure to benzene: People who breathe in high levels of benzene may develop drowsiness, dizziness, rapid or irregular heartbeat, headaches, tremors, confusion unconsciousness, death. Eating foods or drinking beverages containing high levels of benzene can cause vomiting, irritation of the stomach, dizziness, sleepiness, convulsions, rapid or irregular heartbeat, death. Long-term (a year or more) exposure to benzene causes harmful effects on the bone marrow, resulting in anemia and excessive bleeding. It can also affect the immune system, increasing the chance for infection. Some women who breathed high levels of benzene for many months had irregular menstrual periods and a decrease in the size of their ovaries. Acute deaths from benzene exposure at high concentrations have been due to ventricular fibrillation caused by exertion and release of epinephrine. Benzene causes cancer in humans. A retrospective cohort study was conducted in 233 benzene factories and 83 control factories in 12 cities in China. The benzene cohort and the control cohort consisted of 28,460 benzene exposed workers and 28,257 control workers. The leukemia mortality rate was 14/100,000 person-years in the benzene cohort and 2/100,000 person-years in the control cohort. Most (76.6%) cases of benzene leukemia were of the acute type. The mortality due to benzene leukemia was high in organic synthesis plants followed by painting and rubber synthesis industries. The concentration of benzene to which patients with a leukemia were exposed ranged from 10 to 1000 mg/cu m (mostly from 50 to 500 mg/cu m). Benzene is genotoxic in humans: a significantly increased frequency of chromatid and isochromatid breaks in the cultured lymphocytes of exposed workers has been reported, as well as a significant increase of peripheral blood lymphocyte chromosomal aberrations. Metabolic activation of benzene by rat liver microsomes induced sister chromatid exchanges and cell division delays in cultured human lymphocytes. Occupational exposure to benzene may occur through inhalation and dermal contact. The general population may be exposed to benzene via inhalation of ambient air, ingestion of food and drinking water, and dermal contact with consumer products containing benzene. ANIMAL TOXICITY STUDIES: Experimental animal studies, both inhalation and oral, also support the evidence that exposure to benzene increases the risk of cancer in multiple organ systems, including the hematopoietic system, oral and nasal cavities, liver, forestomach, preputial gland, lung, ovary, and mammary gland. Rats exposed to 3,526-8,224 ppm of benzene in a closed chamber for 15 minutes exhibited an increased number of ectopic ventricular beats. In developmental study, rats exposed to 10, 50, or 500 ppm (32, 160 & 1600 mg/cu m) of benzene for 7 hr/day had low incidence of brain and skeletal defects. Rats exposed continuously to 209.7 ppm for 10 days prior to breeding showed a complete absence of pregnancy. 1/10 rats exposed to 19.8 ppm had resorbed embryos. Genotoxicity studies have demonstrated the induction of chromosomal aberrations in bone-marrow cells from mice, rats, and rabbits treated with single or multiple daily doses of benzene ranging from about 0.2 to 2.0 mL/kg per day given either sc or ip. The major metabolites of benzene are phenol, hydroquinone, and catechol. The route of exposure has little effect on the subsequent metabolism of benzene to hemotoxic metabolites. ECOTOXICITY STUDIES: Young Coho salmon mortality was 12/20 at 50 ppm after 24 hr up to 96 hr and 30/30 at 100 ppm after 24 hr in artificial seawater at 8 °C. Herring and anchovy larvae studies showed that 35-45 ppm caused delay in development of eggs and produced abnormal larvae; 10-35 ppm caused delay in development of larvae, decrease in feeding and growth, and increase in respiration. Blue crab juveniles when exposed to sublethal concentrations of benzene (0.1 or 5.0 ppm) in a static system showed an increase in the time needed to complete a molt cycle (50 days in case of benzene-exposed crab, as compared to 33 days for controls), a slower rate of growth of regenerating limb buds, and a depressed activity of ATPase in mitochrondria. Oxygen consumption by the crab decreased from exposure to 1.0 ppm benzene.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
苯的有毒成分是其代谢物。苯能够通过诱导其主要的代谢酶细胞色素P450 2E1来增加其毒性。苯的主要毒性效应是血液细胞计数和骨髓细胞密度的降低。血细胞计数的减少可能是由于代谢物如苯酚与血液蛋白白蛋白和血红蛋白的结合。在骨髓中,酚类代谢物可以被骨髓过氧化物酶代谢为高度反应性的半醌自由基和醌类,这些物质刺激活性氧种类的产生。这一点以及直接的代谢物结合导致微管蛋白、组蛋白和拓扑异构酶II的损伤。一些代谢物还通过抑制其他与DNA相关的蛋白,如线粒体DNA聚合酶和核糖核苷酸还原酶,以及与DNA本身共价结合,产生突变效应,导致如链断裂、有丝分裂重组、染色体易位和非整倍体等效应。
The toxic agents of benzene are its metabolites. Benzene is able increase its toxicity by inducing cytochrome P450 2E1, its main metabolic enzyme. Benzene's primary toxic effects are decreases in haematological cell counts and bone marrow cellularity. The decrease in blood cell count may be due to the binding of metabolites such as benzene oxide to the blood proteins albumin and haemoglobin. In the bone marrow, phenolic metabolites can be metabolized by bone marrow peroxidases to highly reactive semiquinone radicals and quinones that stimulate the production of reactive oxygen species. This and direct metabolite binding leads to damage to tubulin, histone proteins, and topoisomerase II. Some metabolites also exert mutagenic effects by inhibiting other DNA associated proteins, such as mitochondrial DNA polymerase and ribonucleotide reductase, as well as covalently binding to DNA itself, causing effects such as strand breakage, mitotic recombination, chromosome translocations, and aneuploidy. (L5)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
致癌性分类:1)人类证据:充分;2)动物证据:充分;对人类致癌风险的总体评估为第1组:该化学物质对人类致癌。/来自表格/
Classification of carcinogenicity: 1) evidence in humans: sufficient; 2) evidence in animals: sufficient; Overall summary evaluation of carcinogenic risk to humans is group 1: The chemical is carcinogenic to humans. /From table/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A1; 已确认的人类致癌物。
A1; Confirmed human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
癌症分类:对人类致癌
Cancer Classification: Carcinogenic to Humans
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
苯很容易通过肺部吸收,大约有40-50%被保留。它优先被脂肪和神经组织吸收,大约有30-50%不经改变通过肺部排出;在约0.7-1.7小时、3-4小时和20-30小时可以看到一个三阶段的排泄模式。
Benzene is readily absorbed via lung, & about 40-50% is retained. ... It is taken up preferentially by fatty & nervous tissues, & about 30-50% ... is excreted unchanged via lung; a 3-phase excretion pattern is seen at ... /approx/ 0.7-1.7 hr, 3-4 hr, & 20-30 hr.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
当苯置于封闭杯下方的皮肤上时,它以0.4毫克/平方厘米/小时的速度被吸收(Hanke等人,1961年)...
When benzene was placed on skin under closed cup it was absorbed at rate of 0.4 mg/sq cm/hr (Hanke et al 1961) ...
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
以3H标记的苯以2 mL/kg皮下注射处理的小鼠,在以下器官中发现了不可逆结合的放射性,结合量随器官不同而减少:肝脏、大脑、肾脏、脾脏、脂肪。每天以0.5 mL/kg的3H-苯皮下注射处理两次,持续1-10天的小鼠,肝脏和骨髓残留物的放射性结合随着处理时间的增加而增加,除了骨髓的结合量在第六天后开始减少。
Mice treated SC with 2 mL (3)H-labeled benzene/kg contained irreversibly bound radioactivity with decreasing binding magnitude in the following organs: liver, brain, kidney, spleen, fat. Mice treated with 2 daily SC doses of 0.5 mL (3)H-benzene/kg for 1-10 days showed a radioactivity binding with liver & bone marrow residues which increased with treatment duration, except in the case of binding to bone marrow which decreased after day 6.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
当以皮下注射的方式给予小鼠时,72%的剂量会在呼出的空气中回收。
When administered to mice subcutaneously, 72% of dose is recovered in expired air.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:E
-
职业暴露限值:TWA: 0.1 ppm, STEL: 1 ppm
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:500 ppm
-
危险品标志:F
-
安全说明:S36/37,S45,S53
-
危险类别码:R36/38,R45,R48/23/24/25,R11,R65,R46
-
WGK Germany:3
-
海关编码:2707100000
-
危险品运输编号:UN 1114 3/PG 2
-
危险类别:3
-
RTECS号:CY1400000
-
包装等级:II
-
危险标志:GHS02,GHS07,GHS08
-
危险性描述:H225,H304,H315,H319,H340,H350,H372,H412
-
危险性防范说明:P201,P210,P280,P308 + P313,P370 + P378,P403 + P235
-
储存条件:储存注意事项: - 储存在阴凉、通风良好的库房中。 - 远离火源和热源,库温不宜超过37℃。 - 保持容器密封。 - 应与氧化剂及食用化学品分开存放,切忌混储。 - 使用防爆型照明和通风设施。 - 禁止使用可能产生火花的机械设备和工具。 - 储区应配备泄漏应急处理设备和合适的收容材料。
SDS
模块 1. 化学品
1.1 产品标识符
:苯
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
易燃液体 (类别 2)
无可观察效应浓度 - 肥头鲦鱼 (黑头软口鲦鱼) - 10.2 mg/l - 7 d
LOEC - 肥头鲦鱼 (黑头软口鲦鱼) - 17.2 mg/l - 7 d
对水蚤和其他水生无脊 半数效应浓度(EC50) - 大型蚤 (水蚤) - 22.00 mg/l - 48 h
椎动物的毒性
半数效应浓度(EC50) - 大型蚤 (水蚤) - 9.20 mg/l - 48 h
对藻类的毒性 半数效应浓度(EC50) - 近头状伪蹄形藻 (绿藻) - 29.00 mg/l - 72 h
12.2 持久性和降解性
生物降解能力 结果: - 易生物降解。
12.3 潜在的生物累积性
生物富集或生物积累性 高体雅罗鱼 (金雅罗鱼) - 3 d -0.05 mg/l
生物富集因子 (BCF): 10
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
对水生生物有毒。
模块 13. 废弃处置
13.1 废物处理方法
产品
在装备有加力燃烧室和洗刷设备的化学焚烧炉内燃烧处理,特别在点燃的时候要注意,因为此物质是高度易燃
性物质 将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 1114 国际海运危规: 1114 国际空运危规: 1114
14.2 联合国运输名称
欧洲陆运危规: BENZENE
国际海运危规: BENZENE
国际空运危规: Benzene
14.3 运输危险类别
欧洲陆运危规: 3 国际海运危规: 3 国际空运危规: 3
14.4 包裹组
欧洲陆运危规: II 国际海运危规: II 国际空运危规: II
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
急性毒性, 经口 (类别 5)
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
生殖细胞致突变性 (类别 1B)
致癌性 (类别 1A)
吸入危险 (类别 1)
急性水生毒性 (类别 2)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H225 高度易燃液体和蒸气
H303 吞咽可能有害。
H304 吞咽并进入呼吸道可能致命。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H340 可能导致遗传性缺陷。
H350 可能致癌。
H401 对水生生物有毒。
警告申明
预防措施
P201 在使用前获取特别指示。
P202 在读懂所有安全防范措施之前切勿操作。
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P233 保持容器密闭。
P240 容器和接收设备接地。
P241 使用防爆的电气/ 通风/ 照明 设备。
P242 只能使用不产生火花的工具。
P243 采取措施,防止静电放电。
P264 操作后彻底清洁皮肤。
P273 避免释放到环境中。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
事故响应
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P303 + P361 + P353 如果皮肤(或头发)接触:立即除去/脱掉所有沾污的衣物,用水清洗皮肤/淋
浴。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P308 + P313 如接触到或有疑虑:求医/ 就诊。
P321 具体处置(见本标签上提供的急救指导)。
P331 不要诱发呕吐。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
安全储存
P403 + P235 保持低温,存放于通风良好处。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
只限于专业使用者。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C6H6
分子式
: 78.11 g/mol
分子量
组分 浓度或浓度范围
Benzene
<=100%
化学文摘登记号(CAS 71-43-2
No.) 200-753-7
EC-编号 601-020-00-8
索引编号 01-2119447106-44-XXXX
注册号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
恶心, 头晕, 头痛, 麻醉,
吸入高浓度的苯首先是中枢神经系统效应,表现为愉快、神经兴奋和/或眼花、沮丧、困倦或疲劳感。受害者
会经历胸腔发紧、呼吸困难和意识丧失。大量接触后数分钟到数小时会发生震颤、抽搐,甚至因呼吸麻痹和循
环衰竭导致死亡。吸入少量液体立即仪器肺水肿和肺组织出血。直接皮肤接触会引起红斑。长期或反复皮肤接
触会引起脱水、收缩性皮炎、皮肤继发感染。主要的靶器官是造血系统。情况发展会发生流鼻血、牙龈和粘膜
出血、紫瘢、血细胞减少、白细胞减少、血小板减少、贫血和白血病。骨髓可能表现正常、无定形或增生,可
能与外周血形成不同步。长期接触, 血液病
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
无数据资料
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
用水喷雾冷却未打开的容器。
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 移去所有火源。
人员疏散到安全区域。 谨防蒸气积累达到可爆炸的浓度。蒸气能在低洼处积聚。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
围堵溢出,用防电真空清洁器或湿刷子将溢出物收集起来,并放置到容器中去,根据当地规定处理(见第13部
分)。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免曝露:使用前需要获得专门的指导。避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
组分 化学文摘登 值 容许浓度 基准
记号(CAS
No.)
Benzene PC- 6 mg/m3 工作场所有害因素职业接触限值 -
TWA 化学有害因素
备注 确认人类致癌物
皮
PC- 10 mg/m3 工作场所有害因素职业接触限值 -
STEL 化学有害因素
确认人类致癌物
皮
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 氟橡胶
最小的层厚度 0.7 mm
溶剂渗透时间: 480 min
测试过的物质VitojECt® (KCL 890 / Z677698, 规格 M)
飞溅保护
物料: 氟橡胶
最小的层厚度 0.7 mm
溶剂渗透时间: 480 min
测试过的物质VitojECt® (KCL 890 / Z677698, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 阻燃防静电防护服,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 5.5 °C - lit.
f) 沸点、初沸点和沸程
80 °C - lit.
g) 闪点
-11.0 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 爆炸上限: 8 %(V)
爆炸下限: 1.3 %(V)
k) 蒸气压
221.3 hPa 在 37.7 °C
99.5 hPa 在 20.0 °C
l) 蒸汽密度
无数据资料
m) 密度/相对密度
0.874 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
562.0 °C
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
热,火焰和火花。 极端温度和直接日晒。
10.5 不相容的物质
酸, 碱, 卤素, 强氧化剂, 金属盐类
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 2,990 mg/kg
半数致死浓度(LC50) 吸入 - 大鼠 - 雌性 - 4 h - 44,700 mg/m3
半数致死剂量 (LD50) 经皮 - 兔子 - 8,263 mg/kg
皮肤刺激或腐蚀
皮肤 - 兔子 - 皮肤刺激
眼睛刺激或腐蚀
眼睛 - 兔子 - 眼睛刺激
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
实验室测试表明由诱变效应
活体试验表明有致突变效应
细胞突变性-体外试验 - 人 - 淋巴细胞
姐妹染色单体互换
细胞突变性-体外试验 - 小鼠 - 淋巴细胞
哺乳动物体细胞突变
细胞突变性-体内试验 - 小鼠 - 吸入
姐妹染色单体互换
致癌性
致癌性 - 人 - 雄性 - 吸入
肿瘤发生:符合RTECS标准的致癌性。 白血病 血:血小板减少 症。
致癌性 - 大鼠 - 经口
肿瘤发生:符合RTECS标准的致癌性。 内分泌的:肿瘤 白血病
该产品是或包含被IARC, ACGIH, EPA, 和 NTP 列为致癌物的组分
对人类的致癌物。
IARC:
1 - 第1组:对人类致癌 (Benzene)
生殖毒性
生殖毒性 - 小鼠 - 腹膜内的
对生殖的影响:胚胎植入前死亡率(例如每个雌性的植入胚胎数减少;每个黄体的植入总数。
对胚胎或胎儿的影响:胎儿死亡。
发育毒性 - 大鼠 - 吸入
对胚胎或胎儿的影响:超大胚胎结构(例如胎盘、脐带)。
对胚胎或胎儿的影响:胎儿毒性(死亡除外,例如矮小胎儿)。
发育毒性 - 小鼠 - 吸入
对胚胎或胎儿的影响:细胞学改变(包括体细胞遗传物质)。
特定发育异常:血液和淋巴系统(包括脾和骨髓)。
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
吞咽并进入呼吸道可能致命。
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。 摄入有吸入危害-能进入肺部并引起损伤。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
恶心, 头晕, 头痛, 麻醉,
吸入高浓度的苯首先是中枢神经系统效应,表现为愉快、神经兴奋和/或眼花、沮丧、困倦或疲劳感。受害者
会经历胸腔发紧、呼吸困难和意识丧失。大量接触后数分钟到数小时会发生震颤、抽搐,甚至因呼吸麻痹和循
环衰竭导致死亡。吸入少量液体立即仪器肺水肿和肺组织出血。直接皮肤接触会引起红斑。长期或反复皮肤接
触会引起脱水、收缩性皮炎、皮肤继发感染。主要的靶器官是造血系统。情况发展会发生流鼻血、牙龈和粘膜
出血、紫瘢、血细胞减少、白细胞减少、血小板减少、贫血和白血病。骨髓可能表现正常、无定形或增生,可
能与外周血形成不同步。长期接触, 血液病
附加说明
化学物质毒性作用登记: CY1400000
模块 12. 生态学资料
12.1 生态毒性
对鱼类的毒性 半数致死浓度(LC50) - 虹鳟 (红鳟鱼) - 5.90 mg/l - 96 h
半数致死浓度(LC50) - 肥头鲦鱼 (黑头软口鲦鱼) - 15.00 - 32.00 mg/l - 96 h
半数致死浓度(LC50) - 蓝鳃太阳鱼 - 230.00 mg/l - 96 h
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
从这段详细的文字中,我们可以总结出关于苯的以下重要信息:
-
化学性质:无色至淡黄色易挥发液体,具有强烈芳香气味。易燃有毒。
-
主要用途:
-
生产方法:主要有炼焦副产回收苯、铂重整法和裂解汽油制苯法。
-
安全性:
- 易燃易爆,与空气混合可爆炸
- 有毒,短期吸入高浓度可能致死
- 长期接触可能导致健康问题
-
职业卫生标准:短时间接触允许暴露量( STEL)20毫克/立方米; 时间加权平均容许浓度(TWA)16毫克/立方米。
-
存储和运输注意事项:
- 库房通风干燥低温
- 与氧化剂分开存放
- 灌装时流速不超过3米/秒
- 灭火方式:干粉、干砂、二氧化碳、泡沫等
这段文字提供了苯的化学特性、用途、生产工艺、安全性和储存运输信息,为了解和使用这种重要化工原料提供了全面的基础知识。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯-13C6 benzene-(13)C6 32488-44-1 C6H6 84.0476 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 环己-1,3-二烯-5-炔 benzyne 462-80-6 C6H4 76.0978
反应信息
-
作为反应物:描述:参考文献:名称:Symons; Zincke, Justus Liebigs Annalen der Chemie, 1874, vol. 171, p. 131摘要:DOI:
-
作为产物:描述:(benzyloxy)benzene 在 2-吡啶甲酸 、 potassium phosphate 、 copper(l) iodide 、 (TMEDA)Ni(CH2TMS)2 、 氢气 、 sodium t-butanolate 作用下, 以 二甲基亚砜 、 间二甲苯 为溶剂, 100.0~120.0 ℃ 、100.0 kPa 条件下, 反应 212.0h, 生成 苯参考文献:名称:一种用于芳醚氢解而不用芳烃加氢的多相镍催化剂摘要:描述了一种用于芳醚选择性氢解生成芳烃和醇的多相镍催化剂,无需添加配位体。催化剂由明确的可溶性镍前体 Ni(COD)(2) 或 Ni(CH(2)TMS)(2)(TMEDA) 在碱性添加剂如 (t)BuONa 存在下原位形成. 该催化剂选择性地裂解木质素芳醚模型中的 C(Ar)-O 键,而无需氢化芳环,并且在 1 bar 的 H(2) 压力下,它的负载量可降至 0.25 mol%。这种催化剂对电子变化的芳醚的选择性不同于之前报道的均相催化剂,这意味着这两种催化剂彼此不同。DOI:10.1021/ja3085912
-
作为试剂:描述:乙二醇 、 3-benzyl-3'-chloro-4'-methyl-2,2',4,5'-tetraoxo-5-propyl-3-azaspiro[bicyclo[3.1.0]hexane-6,1'-cyclopentan]-3'-ene-1-carbaldehyde 在 对甲苯磺酸 、 苯 作用下, 以71 %的产率得到rel-(5S,6S)-3-benzyl-3'-chloro-4'-methyl-2,2',4,5'-tetraoxo-1-(1,3-dioxoranyl)-5-propyl-3-azaspiro[bicyclo[3.1.0]hexane-6,1'-cyclopentan]-3'-ene参考文献:名称:环蠕虫醇X的三环六取代螺环丙烷核的立体选择性制备摘要:本研究的重点是合成环蠕虫醇 X ( 1 ) 的三环六取代螺环丙烷核心框架2 ,环蠕虫醇 X 是一种以立体选择性方式从Helminthosporium velutinum yone96 中分离出来的抗真菌细胞毒素。该合成的特点是通过 8-氮杂三环[4.3.0.1 2,5 ]deca-3,7,9-trione 衍生物的逆迈克尔型开环反应生成氯化季盐23的 S N 2 型环丙烷化反应22 .成功的合成证实了1的结构,解决了由于缺乏X射线晶体学分析而产生的歧义。制备的模型表现出有效的细胞毒性。DOI:10.1021/acs.orglett.4c00203
文献信息
-
Condensed-Phase, Halogen-Bonded CF<sub>3</sub>I and C<sub>2</sub>F<sub>5</sub>I Adducts for Perfluoroalkylation Reactions作者:Filippo Sladojevich、Eric McNeill、Jonas Börgel、Shao-Liang Zheng、Tobias RitterDOI:10.1002/anie.201410954日期:2015.3.16family of practical, liquid trifluoromethylation and pentafluoroethylation reagents is described. We show how halogen bonding can be used to obtain easily handled liquid reagents from gaseous CF3I and CF3CF2I. The synthetic utility of the new reagents is exemplified by a novel direct arene trifluoromethylation reaction as well as adaptations of other perfluoroalkylation reactions.
-
葡萄糖苷的二环衍生物及其制备方法和用途
-
[EN] SULFONYL COMPOUNDS THAT INTERACT WITH GLUCOKINASE REGULATORY PROTEIN<br/>[FR] COMPOSÉS DE SULFONYLE QUI INTERAGISSENT AVEC LA PROTÉINE RÉGULATRICE DE LA GLUCOKINASE申请人:AMGEN INC公开号:WO2013123444A1公开(公告)日:2013-08-22The present invention relates to sulfonyl compounds that interact with glucokinase regulatory protein. In addition, the present invention relates to methods of treating type 2 diabetes, and other diseases and/or conditions where glucokinase regulatory protein is involved using the compounds, or pharmaceutically acceptable salts thereof, and pharmaceutical compositions that contain the compounds, or pharmaceutically acceptable salts thereof.
-
一种3,5-二甲氧基苯甲酸乙酯的合成方法申请人:丁玉琴公开号:CN105218322A公开(公告)日:2016-01-06本发明公开了一种3,5-二甲氧基苯甲酸乙酯的合成方法,属于化学合成领域。本发明先用苯在特定的条件下生产间苯二酚,在和无水碳酸钾和二氧化碳在二甲基甲酰胺为溶剂下,通过除杂、脱色、调节pH值、冷却结晶最终得到3,5-二羟基苯甲酸,再和硫酸二甲酯搅拌的条件下混合,向其中滴加氢氧化钠水溶液进行回流,回流后调节pH值,冷却至0℃减压过滤、洗涤、干燥得到3,5-二甲氧基苯甲酸,最后和无水乙醇在浓硫酸的条件下进行酯化,最终得3,5-二甲氧基苯甲酸乙酯。
-
Anionic chiral cobalt(III) complexes as catalysts of asymmetric synthesis of cyanohydrins作者:Yu. N. Belokon’、V. I. Maleev、I. L. Mal’fanov、T. F. Savel’eva、N. S. Ikonnikov、A. G. Bulychev、D. L. Usanov、D. A. Kataev、M. NorthDOI:10.1007/s11172-006-0338-4日期:2006.5Chiral coordinatively saturated cobalt(III) complexes with Schiff bases of enantio-pure amino acids are formed as Λ and Δ-isomers, which are not transformed into each other under normal conditions. These complexes catalyze the formation of enantiomerically enriched cyanohydrins from aldehydes and Me3SiCN under homo-and heterogeneous catalysis.
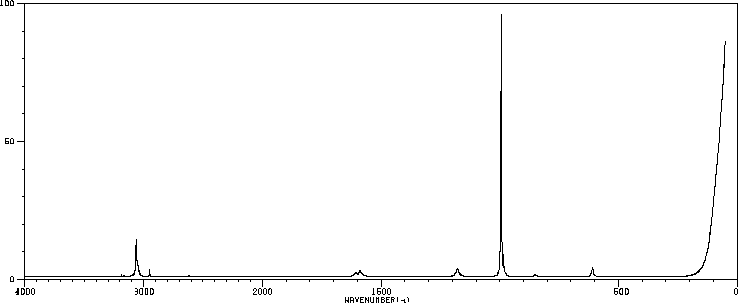
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫