6-chloropurine riboside | 2004-06-0
中文名称
——
中文别名
——
英文名称
6-chloropurine riboside
英文别名
6-chloro-9β-D-ribofuranosyl-9H-purine;6-Chloro-9-ribofuranosyl-9H-purine;(3R,4S,5R)-2-(6-chloropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
CAS
2004-06-0
化学式
C10H11ClN4O4
mdl
——
分子量
286.675
InChiKey
XHRJGHCQQPETRH-VTHZCTBJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:158-162 °C (dec.)(lit.)
-
沸点:614.8±65.0 °C(Predicted)
-
密度:1.7303 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:19
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:114
-
氢给体数:3
-
氢受体数:7
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
海关编码:2942000000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
反应信息
-
作为反应物:描述:6-chloropurine riboside 在 咪唑 、 三丁基氯化锡 、 2,2,6,6-tetramethylpiperidinyl-lithium 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 16.0h, 生成 1-((3R,4R,5R)-2-{6-chloro-2-[1-benzylpyrazol-4-yl]purin-9-yl}-4-(1,1,2,2-tetramethyl-1-silapropoxy)-5-[(1,1,2,2-tetramethyl-1-silapropoxy)methyl]oxolan-3-yloxy)-1,1,2,2-tetramethyl-1-silapropane参考文献:名称:2-Pyrazolyl-N6-Substituted Adenosine Derivatives as High Affinity and Selective Adenosine A3 Receptor Agonists摘要:We describe the synthesis of new high affinity and selective A(3)-adenosine receptor (A(3)-AdoR) agonists. Introduction of a methyl group at the N-6-position of the A(2A)-AdoR selective 2-pyrazolyladenosine analogues (Figure 2) brought about a substantial increase in the A(3)-AdoR binding affinity and selectivity. While the N-6-desmethyl analogues 3a and 4 were inactive at the A(3)AdoR (K-i > 10,muM), the corresponding N-6-methyl analogues 5 and 22 showed good binding affinity at the A(3)-AdoR (K-i = 73 and 97 nM, respectively). Replacement of the carboxamide group in 5 with different heteroaryl groups resulted in analogues with high affinities and selectivity for the A(3)-AdoR. (2R,3S,4R)-tetrahydro-2-(hydroxymethyl)-5-(6-(methylamino)-2-(4-(pyridin-2-yl)-1H-pyrazol-1-yl)-9H-purin-9-yl)furan-3,4-diol (15, Ki = 2 nM) displayed high selectivity for the A(3)-AdoR versus A(1)- and A(2A)-AdoRs (selectivity ratios of 1900 and >2000, respectively).DOI:10.1021/jm049682h
文献信息
-
REAGENTS AND METHODS FOR SIRTUIN CAPTURE申请人:SAUVE Anthony A.公开号:US20130065248A1公开(公告)日:2013-03-14The invention provides a method of preparing a sirtuin complex. The invention also provides a method of detecting a sirtuin in a sample comprising use of the aforesaid sirtuin complex.这项发明提供了一种制备sirtuin复合物的方法。该发明还提供了一种检测样品中sirtuin的方法,包括使用上述sirtuin复合物。
-
[EN] NOVEL HISTONE METHYLTRANSFERASE INHIBITORS<br/>[FR] NOUVEAUX INHIBITEURS D'HISTONE MÉTHYLTRANSFÉRASES申请人:UNIV FREIBURG ALBERT LUDWIGS公开号:WO2021053158A1公开(公告)日:2021-03-25The present invention relates to novel compounds of formula (I) as defined herein. The compounds are inhibitors of histone methyltransferases of the seven-beta-strand family, in particular of KMT9.
-
[EN] NEW BORANOPHOSPHATE ANALOGUES OF CYCLIC NUCLEOTIDES<br/>[FR] NOUVEAUX ANALOGUES BORANOPHOSPHATES DE NUCLÉOTIDES CYCLIQUES申请人:BIOLOG LIFE SCIENCE INST FORSCHUNGSLABOR UND BIOCHEMICA VERTRIEB GMBH公开号:WO2012130829A1公开(公告)日:2012-10-04The present invention relates to novel boranophosphate analogues of cyclic nucleotides. The invention further relates to the use of such compounds as reagents for signal transduction research or as modulators of cyclic nucleotide-regulated binding proteins and isoenzymes thereof, and/or as hydrolysis- and oxidation-resistant ligands for affinity chromatography, for antibody production or for diagnostic applications e.g. on chip surfaces and/or as additive for organ transplantation storage solutions.
-
COMPOUND USED AS AUTOPHAGY REGULATOR, AND PREPARATION METHOD THEREFOR AND USES THEREOF申请人:WIGEN BIOMEDICINE TECHNOLOGY (SHANGHAI) CO., LTD.公开号:US20200190066A1公开(公告)日:2020-06-18It is related to compounds used as autophagy modulators and a method for preparing and using the same, specifically providing a compound of general formula (I), or pharmaceutically acceptable salts thereof, which is a type of autophagy modulators, particularly mammalian ATG8 homologues modulators.该内容涉及作为自噬调节剂的化合物及其制备和使用方法,具体提供了一类具有通用公式(I)的化合物,或其药用可接受盐,这是一种自噬调节剂,特别是哺乳动物ATG8同源物调节剂。
-
[EN] NON-NUCLEOTIDE COMPOSITIONS AND METHOD FOR TREATING PAIN<br/>[FR] COMPOSITIONS NON-NUCLEOTIDIQUES ET PROCEDE DE TRAITEMENT DE LA DOULEUR申请人:INSPIRE PHARMACEUTICALS INC公开号:WO2005039590A1公开(公告)日:2005-05-06The present invention is directed to a method of treating pain. The method comprises administering to a subject a pharmaceutical composition comprising an effective amount of a P2X receptor antagonist. The methods of the present invention are useful in reducing pain, such as traumatic pain, neuropathic pain, organ pain and/or pain associated with diseases. The P2X receptor antagonists useful for this invention are non-nucleotide compounds of general Formula I. Compounds of Formula I can be used alone to treat pain. Compounds of Formula I can also be used in conjunction with other therapeutic agents or adjunctive therapies commonly used to treat pain, thus enhancing the therapeutic effect of pain reduction.本发明涉及一种治疗疼痛的方法。该方法包括向受试者施用含有有效量P2X受体拮抗剂的药物组合物。本发明的方法对于减轻疼痛,如创伤性疼痛、神经病性疼痛、器官疼痛和/或与疾病相关的疼痛具有用处。本发明中用于该发明的P2X受体拮抗剂是一般式I的非核苷化合物。式I的化合物可以单独用于治疗疼痛。式I的化合物也可以与其他治疗剂或常用于治疗疼痛的辅助疗法结合使用,从而增强减轻疼痛的治疗效果。
表征谱图
-
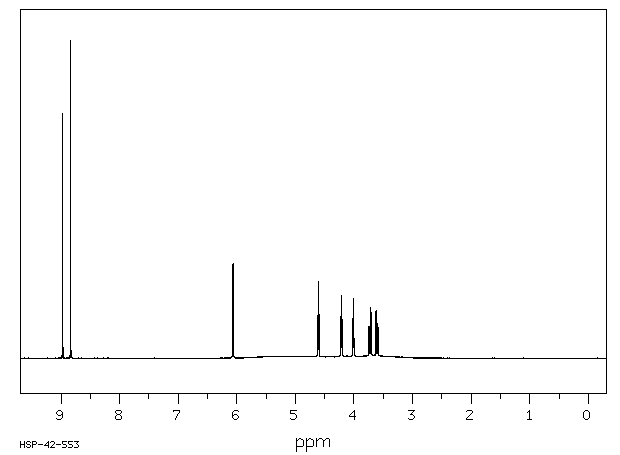
氢谱1HNMR
-
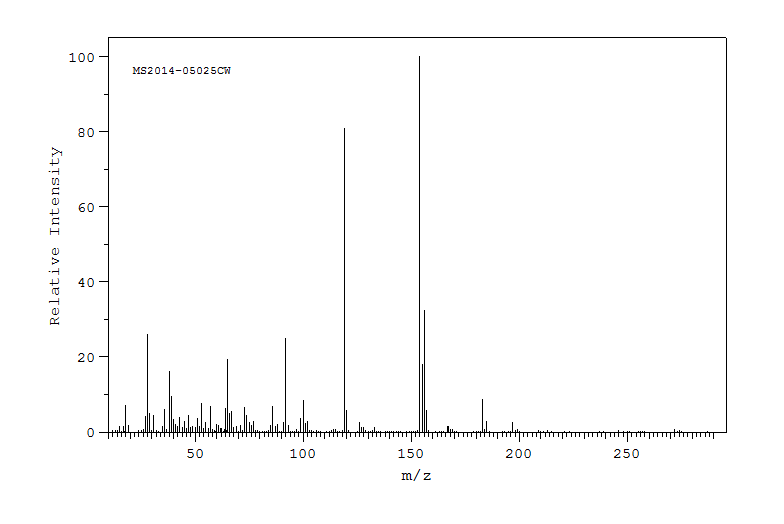
质谱MS
-
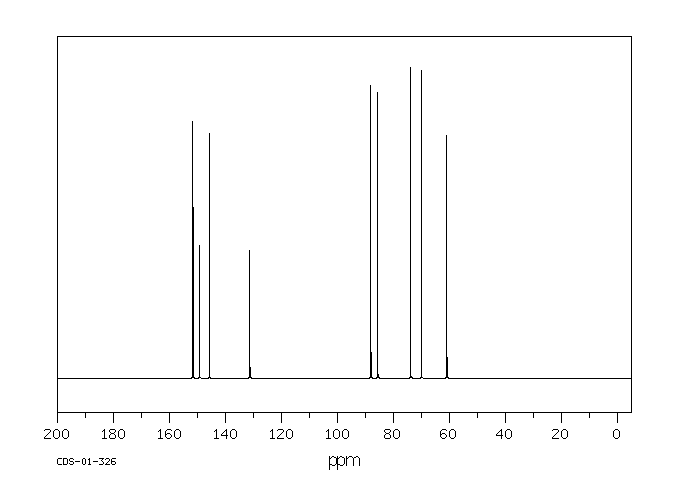
碳谱13CNMR
-
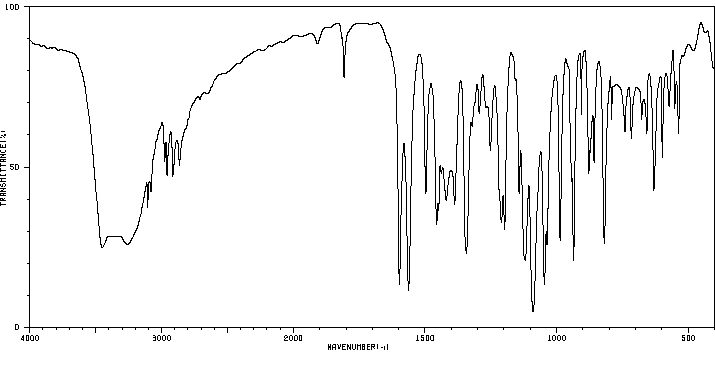
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄质核苷
黄嘌呤核苷
鸟苷5'-硫代二磷酸酯
鸟苷,N,N-二甲基-6-O-[2-(4-硝基苯基)乙基]-
鸟苷 2',3',5'-三苯甲酸酯
鸟苷
马兜铃内酰胺A
顺式-玉米素-D-核糖甙
阿糖腺苷2',3',5'-三乙酸酯
阿糖腺苷 2',3'-二乙酸酯
阿糖腺苷
阿糖腺苷
阿糖肌苷
阿洛酮糖腺苷
阿斯卡霉素
虫草素
苯酰胺,N-[9-[2-脱氧-2-氟-3,5-二-O-(四氢-2H-吡喃-2-基)-β-D-呋喃阿拉伯糖基]-9H-嘌呤-6-基]-
苯酚,4-[4-(2-氨基乙基)-2-碘苯氧基]-,盐酸(1:1)
苏云金素
腺苷酸-5-羧酸
腺苷胺类
腺苷地尔
腺苷6-N-氨基磷酸酯
腺苷5-O-硫一磷酸二锂盐
腺苷5'-O-(1-硫代二磷酸酯)
腺苷-N(6)-二乙硫基醚-N'-吡啶氧杂亚胺5'-磷酸酯
腺苷-5'-羧酸2-氯乙酯
腺苷-5'-单烟酸酯
腺苷-5'-13C
腺苷-3(+2')-单磷酸单水合物
腺苷-2,8-T2
腺苷-2'-13C
腺苷-15NN1-氧化物
腺苷,8-(丁基氨基)-N-环戊基-
腺苷,2-溴-
腺苷,2-氯-N-环丙基-
腺苷,2-[(4-甲代戊基)氧代]-
腺苷,2-(苯基甲氧基)-
腺苷,2-(环己三烯并氧基)-
腺苷,2-(2-乙基丁氧基)-
腺苷,2',3'-O-(1-甲基亚乙基)-,5'-氨基磺酸酯
腺苷
肌苷肟
肌苷-8-14C
肌苷
美他卡韦
硒-(4-硝基苯甲酰基)-6-硒肌苷
甲基4,5-二溴-3-氟噻吩-2-羧酸酯
瑞加德松杂质3
瑞加德松杂质1