L-谷氨酸脱氢酶 | 2486-02-4
中文名称
L-谷氨酸脱氢酶
中文别名
没食子酸异戊酯
英文名称
isopentyl 3,4,5-trihydroxybenzoate
英文别名
3-methylbutyl gallate;isoamyl gallate;3,4,5-trihydroxy-benzoic acid isopentyl ester;3,4,5-Trihydroxy-benzoesaeure-isopentylester;Gallussaeure-isopentylester;Gallussaeureisoamylester;3-methylbutyl 3,4,5-trihydroxybenzoate
CAS
2486-02-4;9029-11-2
化学式
C12H16O5
mdl
MFCD00016431
分子量
240.256
InChiKey
YBMTWYWCLVMFFD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:142°C
-
沸点:450.9±40.0 °C(Predicted)
-
密度:1.271±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:17
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.416
-
拓扑面积:87
-
氢给体数:3
-
氢受体数:5
安全信息
-
安全说明:S22,S24/25
-
海关编码:2918290000
SDS
制备方法与用途
概述
没食子酸与醇反应生成的没食子酸酯类化合物被广泛应用在食品、化工、轻工和医药等行业。其中,没食子酸丙酯和没食子酸辛酯是我国批准使用的油脂类食品抗氧化剂,并且相关研究较多。相比之下,作为没食子酸衍生物之一的没食子酸异戊酯的研究较少。因此,探究没食子酸异戊酯的合成及其对生物柴油抗氧化性能的研究具有重要意义。
应用没食子酸酯是没食子酸的重要衍生物,也是一种安全高效的食品抗氧化剂,并且能有效抑制细菌、酵母和霉菌等微生物的生长。它对人体内自由基有强烈的清除作用,具备延缓衰老和预防心血管疾病的功能。没食子酸异戊酯作为一种脂溶性抗氧化剂,不仅能治疗心脑血管疾病和清除体内自由基,还对炎症、病毒性疾病、辐射损伤、过敏及衰老等方面具有一定的疗效。因此,在化工、轻工、医药和食品等行业中有着广泛的应用前景,可用于食品抗氧化剂、新型感光材料的添加剂以及牙膏中的抗菌成分。
制备在50 mL三口烧瓶中加入没食子酸1.70 g(0.01 mol),一定量的异戊醇,并添加对甲苯磺酸。放入磁性搅拌子,插入温度计并安装回流装置。开启磁力搅拌,于不同温度下油浴加热并回流一段时间。反应完成后,将反应液转移至分液漏斗中,加入等体积的饱和碳酸氢钠溶液洗涤有机相,以除去未反应的没食子酸,并水洗直至中性。通过减压蒸馏去除多余的醇(可回收利用),向剩余固体中加入适量氯仿并趁热过滤。待氯仿完全挥发后,用乙酸乙酯溶解沉淀物,再进行过滤,收集滤液并通过减压浓缩和干燥后得到产品。
化学性质没食子酸异戊酯是一种白色至浅棕黄色的结晶固体,无臭味,略带微苦。其熔点范围为140~145℃,不溶于水但易溶于乙醇中。
用途主要用于食品抗氧化剂。
生产方法上下游信息
反应信息
-
作为反应物:描述:L-谷氨酸脱氢酶 在 Tris-HCl buffer 、 乙二胺四乙酸 、 双(4-硝基苯基)硫酸盐 、 维生素 C 作用下, 以 水 为溶剂, 反应 8.0h, 生成 isoamyl gallate 3-O-sulfate 、 isoamyl gallate 4-O-sulfate参考文献:名称:Enzymatic Sulfation of Isoamyl Gallate and (-)-Epigallocatechin Gallate by Bacterial Arylsulfotransferase.摘要:从人类肠道细菌中获得的一种新型芳基硫基转移酶可催化与单宁酸有关的多酚硫酸化。研究发现,以对硝基苯硫酸盐为供体底物,没食子酸异戊酯和(-)-表没食子儿茶素没食子酸酯可被快速硫酸化。就没食子酸异戊酯而言,当等摩尔量的对硝基苯基硫酸盐与没食子酸异戊酯孵育时,可分离出两种硫酸化产物,即 3-单硫酸盐和 4-单硫酸盐。对于(-)-表没食子儿茶素没食子酸酯。在供体和受体等摩尔培养的情况下,分离出 4'-单硫酸盐。因此,芳基硫转移酶有助于方便地制备这些多酚的硫酸酯。DOI:10.1248/cpb.40.1864
-
作为产物:参考文献:名称:没食子酸烷基酯:杀锥虫和杀利什曼原虫活性,以及通过建模研究进行目标识别摘要:通过 Fischer 酯化合成了八种没食子酸烷基酯 ( 1–8 ),并使用布氏锥虫和大利什曼原虫前鞭毛体的血流形式评估了它们的锥虫和利什曼虫杀灭活性。用人 HL-60 细胞评估酯的一般细胞毒性。这些化合物表现出中度至良好的杀锥虫活性,但具有零至低的杀利什曼原虫活性。具有线性排列的三个或四个碳原子的烷基链的没食子酸酯(丙基(4),丁基(5)和异戊基(6)) 被发现是最具杀锥虫性的化合物,其 50% 生长抑制值为 ~3 μM。另一方面,HL-60 细胞对这些化合物不太敏感,因此,酯类 4 – 6 的选择性指数(细胞毒性与杀锥虫活性的比率) > 20。结合分子对接和分子动力学模拟的建模研究表明,没食子酸烷基酯的杀锥虫作用机制可能与抑制T. brucei有关替代氧化酶。这一建议得到观察结果的支持,即在甘油作为唯一底物存在的情况下,当与酯一起孵育时,锥虫会在几分钟内变得不动。这些结果表明没食子酸烷基DOI:10.3390/molecules27185876
文献信息
-
Design, Synthesis, and Antifungal Activity of Alkyl Gallates Against Plant Pathogenic Fungi In Vitro and In Vivo作者:Xiao-Long Zhao、Chun-Qing Li、Xiao-Mei Song、Shuang-Mei Yan、Du-Qiang LuoDOI:10.1007/s10600-021-03276-3日期:2021.1A series of alkyl gallates was synthesized by reacting gallic acid with the corresponding alcohols. Their structures were determined on the basis of spectroscopic data, including NMR and MS. The antifungal activities of these compounds against plant pathogenic fungi in vitro and in vivo were assessed.
-
STABILIZATION OF HOUSEHOLD, BODY-CARE AND FOOD PRODUCTS BY USING BENZOTROPOLONE CONTAINING PLANT EXTRACTS AND/OR RELATED BENZOTROPOLONE DERIVATIVES申请人:Wagner Barbara公开号:US20120230925A1公开(公告)日:2012-09-13Disclosed is the use of benzotropolone derivatives of formula (1), wherein R 1 , R 2 and R 7 independently from each other are hydrogen; C 1 -C 3 alkyl; or COR 8 ; R 3 is hydrogen; or COOR 9 R 4 is hydrogen; or C 1 -C 3 alkyl; R 5 is hydrogen; hydroxy; C 1 -C 3 -alkoxy; or -0-(CO)—R 10 ; R 6 is hydrogen; C 1 -C 3 alkyl; or COR 8 ; or R 5 and R 6 together may form a five or six membered ring; or R 6 and R 7 together form a five or six membered ring; and R 8 , R 9 , R 10 independently of each other are C 1 -C 30 alkyl; for protecting body-care and household products from photolytic and oxidative degradation.
-
Microwave-Assisted Esterification of Gallic Acid作者:Zhi-Hao Shi、Nian-Guang Li、Yu-Ping Tang、Qian-Ping Shi、Wei Zhang、Peng-Xuan Zhang、Wei Li、Ze-Xi Dong、Jin-Ao DuanDOI:10.14233/ajchem.2015.17906日期:——An efficient synthesis of alkyl gallates under microwave irradiation was described. The reaction took place in 6-10 mins, which was much shorter than the traditional synthetic methods, with almost quantitative yields.描述了微波辐射下没食子酸烷基酯的有效合成。反应时间为6-10分钟,比传统合成方法短得多,收率几乎定量。
-
Synthesis and in Vitro Antimalarial Activity of Alkyl Esters Gallate as a Growth Inhibitors of Plasmodium Falciparum作者:Ade Arsianti、Hendry Astuti、Fadilah Fadilah、Daniel Martin Simadibrata、Zoya Marie Adyasa、Daniel Amartya、Anton Bahtiar、Hiroki Tanimoto、Kiyomi KakiuchiDOI:10.13005/ojc/340207日期:2018.4.28Malaria is an infectious disease caused by parasite Plasmodium which has a high prevalence in tropical and subtropical countries. In Indonesia, around 396 districts are found as endemic area for malaria, with the highest Annual Parasitic Incidence (API) in Papua, West Papua and East Nusa Tenggara. Currently, treatment for malaria caused by parasite Plasmodium falciparum relies on the use of 4-aminoquinoline or synergistic combinations of antifolates and sulfamethoxazole-trimethoprim. However, this treatment is no longer effective and develop resistancy. This fact suggesting the new antimalarial activity that is more effective and safer are needed. Previous research revealed that gallic acid and its alkyl esters derivatives exhibited antimalarial activity. This study is aimed to synthesize alkyl esters gallate and determine its in vitro antimalarial activity against parasite Plasmodium falciparum. Fourteen compounds of alkyl esters gallate were synthesized by esterification of the carboxyl group of gallic acid with a series of alkyl alcohols, as well as methoxylation of the hydroxy groups on the aromatic ring of gallic acid. Antimalarial activity of the synthesized alkyl esters gallate were expressed by IC50 value, with gallic acid as an original compound and artemisin as a positive control. Compared to gallic acid, eleven synthesized compounds of alkyl esters gallate, have a greater antimalarial activity against Plasmodium falciparum. On the other hand, three compounds, that are propyl gallate, butyl gallate and trimethoxy methyl gallate, showed a lower antimalarial activity. Moreover, compared to gallic acid (IC50 : 194.86 mM) and artemisin (IC50 : 0.5 mM), two synthesized compounds of alkyl gallates, namely methyl gallate and hexyl gallate exhibited the stronger antimalarial activity against Plasmodium falciparum, with IC50 value of 0.03 mM and 0.11 mM, respectively. Our result clearly demonstrated that methyl gallate and hexyl gallate as a promising candidate for the new antimalarial agents.疟疾是由疟原虫引起的传染病,在热带和亚热带国家流行。在印度尼西亚,大约 396 个地区被发现为疟疾流行区,其中巴布亚、西巴布亚和东努沙登加拉的年度寄生虫发病率 (API) 最高。目前,由恶性疟原虫引起的疟疾的治疗依赖于使用4-氨基喹啉或抗叶酸剂和磺胺甲恶唑-甲氧苄啶的协同组合。然而,这种治疗不再有效并产生耐药性。这一事实表明需要更有效、更安全的新抗疟活性。先前的研究表明,没食子酸及其烷基酯衍生物具有抗疟活性。本研究旨在合成没食子酸烷基酯并测定其对恶性疟原虫的体外抗疟活性。通过没食子酸的羧基与一系列烷基醇的酯化反应以及没食子酸芳环上的羟基的甲氧基化反应,合成了14种没食子酸烷基酯化合物。以没食子酸为原始化合物,青蒿素为阳性对照,合成的没食子酸烷基酯的抗疟活性以IC50值表示。 与没食子酸相比,合成的十一种没食子酸烷基酯化合物对恶性疟原虫具有更强的抗疟活性。另一方面,没食子酸丙酯、没食子酸丁酯和没食子酸三甲氧基甲酯这三种化合物显示出较低的抗疟活性。此外,与没食子酸(IC50:194.86 mM)和青蒿素(IC50:0.5 mM)相比,两种合成的没食子酸烷基酯化合物,即没食子酸甲酯和没食子酸己酯,对恶性疟原虫表现出更强的抗疟活性,IC50值为0.03 mM,分别为 0.11 mM。我们的结果清楚地表明没食子酸甲酯和没食子酸己酯是新型抗疟药物的有希望的候选者。
-
Recording materials申请人:Kanzaki Paper Manufacturing Company, Ltd.公开号:US04533930A1公开(公告)日:1985-08-06This invention relates to recording materials comprising: (a) an organic phosphorus-iron compound having a bond of PO.sup.- and/or PS.sup.- with Fe.sup.+++ in the molecule and (b) a ligand compound capable of reacting with the organic phosphorus-iron compound to form a complex.
表征谱图
-
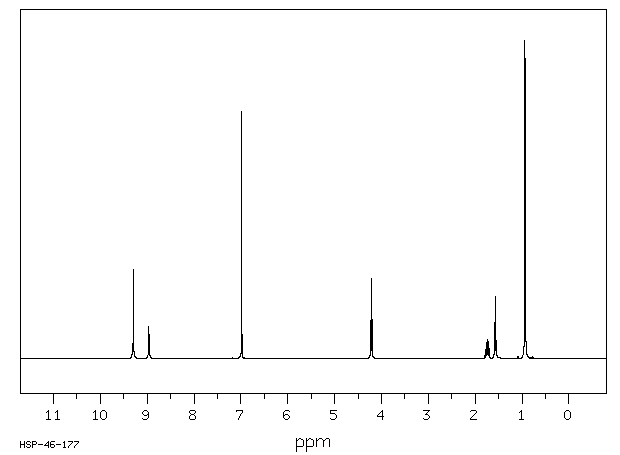
氢谱1HNMR
-
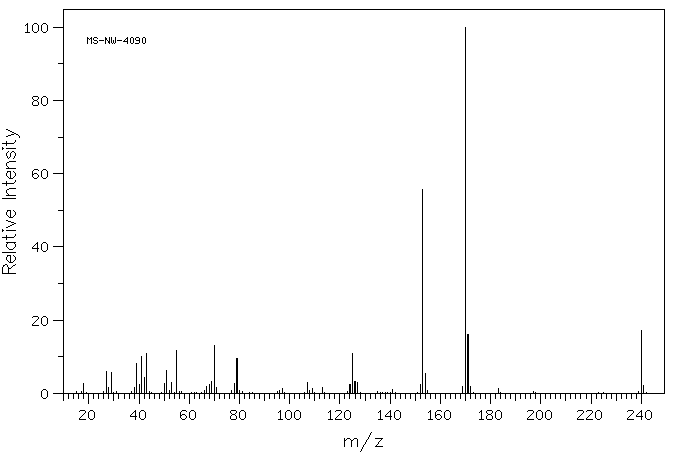
质谱MS
-
碳谱13CNMR
-
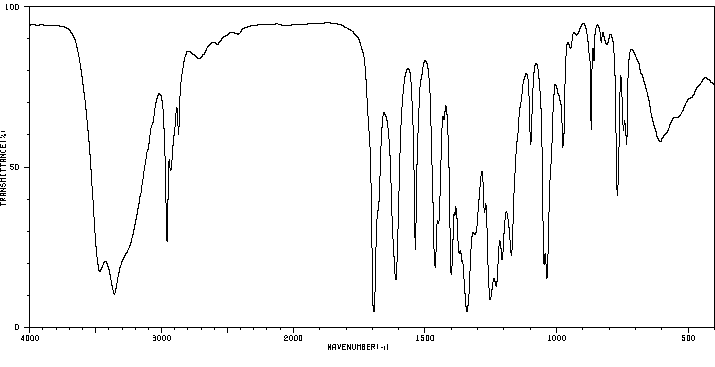
红外IR
-
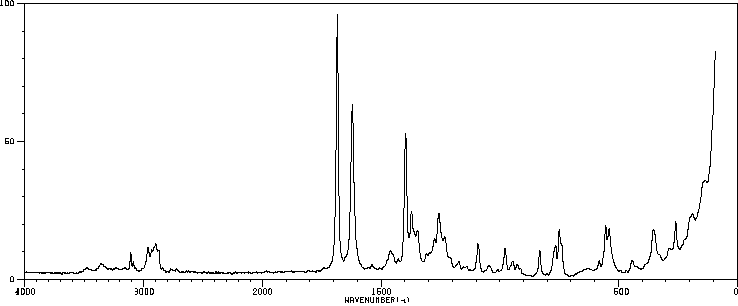
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫