榄香素 | 487-11-6
中文名称
榄香素
中文别名
欖[香]素;5-烯丙基-1,2,3-三甲氧基苯
英文名称
elemicin
英文别名
1,2,3-trimethoxy-5-(2-propenyl)benzene;elemicine;3-(3,4,5-trimethoxyphenyl)-1-propene;5-allyl-1,2,3-trimethoxybenzene;3,4,5-trimethoxyallylbenzene;1,2,3-trimethoxy-5-prop-2-enylbenzene;4-allyl-1,2,6-trimethoxy benzene
CAS
487-11-6
化学式
C12H16O3
mdl
MFCD01656688
分子量
208.257
InChiKey
BPLQKQKXWHCZSS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:152-156 °C
-
密度:1.0630 g/cm3
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
LogP:2.298 (est)
-
物理描述:Colourless to pale straw coloured viscous liquid; Spice with floral notes
-
折光率:1.529-1.534
-
保留指数:1531;1518;1527;1518;1512;1521;1521;1522;1524;1531;1516;1553;1548;1556;1521;1525;1512;1520;1512;1531;1515;1516;1516;1517;1529;1518;1526;1517;1525;1512;1525;1525;1515;1519;1530;1530;1531;1524.2
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
储存条件:存储条件:2-8°C,干燥,密封。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Elemicin
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Elemicin
CAS number: 487-11-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C12H16O3
Molecular weight: 208.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Elemicin
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Elemicin
CAS number: 487-11-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C12H16O3
Molecular weight: 208.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
产品描述
榄香素是一种无色油状液体,沸点为146~147°C/10mmHg。目前,榄香素主要用于医药麻醉。尽管许多植物中含有该成分,但由于含量较低且难以提取,并且杂质较多而被放弃。因此,科研人员尝试采用化学合成的方法来制备榄香素,但这种方法也存在含苯类其他杂质的风险,对人体可能造成较大危害。因此,寻求新的解决方案迫在眉睫。
产品用途榄香素是一种无色油状液体,沸点为146~147°C/10mmHg,目前主要用于医药麻醉。此外,榄香素也可用于制备含有肟菌酯和榄香素的高效杀菌组合物。
生物活性Elemicin 是一种烯基苯类化合物,在许多草药和香料中广泛分布。它通过代谢激活抑制硬脂酰辅酶 A 去饱和酶 1 (SCD1) 的活性。Elemicin 在芳香族食品中的含量较高,具有抗微生物、抗氧化剂和抗病毒的生物活性,并且还具有遗传毒性和致癌性。
靶点- SCD1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 5-allyl-2,3-dimethoxyphenol 76773-99-4 C11H14O3 194.23 4-烯丙基-2,6-二甲氧基苯酚 4-allyl-2,6-dimethoxyphenol 6627-88-9 C11H14O3 194.23 —— 5-Hydroxyeugenol 4055-72-5 C10H12O3 180.203 丁香酚 4-allylguaiacol 97-53-0 C10H12O2 164.204 —— 5-allyl pyrogallol —— C9H10O3 166.177 5-烯丙基-2羟基-3甲氧亚苄基 5-allyl-2-hydroxy-3-methoxybenzaldehyde 22934-51-6 C11H12O3 192.214 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-烯丙基-2,6-二甲氧基苯酚 4-allyl-2,6-dimethoxyphenol 6627-88-9 C11H14O3 194.23 仙人球毒碱 mescaline 54-04-6 C11H17NO3 211.261 —— 1-(3',4',5'-trimethoxyphenyl)propane 41564-88-9 C12H18O3 210.273 3,4,5-三甲氧基苯乙醛 3,4,5-trimethoxyphenylacetaldehyde 5320-31-0 C11H14O4 210.23 3,4,5-三甲氧基苯乙腈 2-(3,4,5-trimethoxyphenyl)acetonitrile 13338-63-1 C11H13NO3 207.229 —— brittonin A 33284-74-1 C20H26O6 362.423 3,4,5-三甲氧基苯乙酸 3,4,5-trimethoxyphenyl acetic acid 951-82-6 C11H14O5 226.229 —— (R)-1-(3,4,5-trimethoxyphenyl)propan-2-ol 835922-46-8 C12H18O4 226.273 —— 1-(3,4,5-trimethoxyphenyl)-2-bromopropane 121637-68-1 C12H17BrO3 289.169 三甲氧他明 3,4,5-trimethoxyamphetamine 1082-88-8 C12H19NO3 225.288 —— 1,4-bis-(3,4,5-trimethoxyphenyl)-2,3-dimethylbutane —— C24H34O6 418.53 —— (S)-3-(3,4,5-trimethoxyphenyl)-1,2-propanediol 638990-29-1 C12H18O5 242.272 —— (2R)-3-(3',4',5'-trimethoxyphenyl)-1,2-propanediol 638990-30-4 C12H18O5 242.272 3-(3',4',5'-三甲氧基苯基)-1,2-丙二醇 p-methoxytodadiol 54306-10-4 C12H18O5 242.272 3,4,5-三甲氧基苯甲醛 3,4,5-trimethoxy-benzaldehyde 86-81-7 C10H12O4 196.203 —— 2-methoxy-5-(3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)cyclohepta-2,4,6-trien-1-one 1616515-30-0 C20H22O5 342.392 —— (R)-1-(3,4,5-trimethoxyphenyl)-2-O-(4-allyl-2,6-dimethoxyphenyl)-2-propanol 124151-41-3 C23H30O6 402.488 —— (S)-1-(3,4,5-trimethoxyphenyl)-2-O-(4-allyl-2,6-dimethoxyphenyl)-2-propanol —— C23H30O6 402.488 1,2,3-三甲氧基-5-((E)-丙-1-烯基)苯 isoelemicin 5273-85-8 C12H16O3 208.257 —— 1,2,3-trimethoxy5(prop1en1yl)benzene 487-12-7 C12H16O3 208.257 异榄香素 Isoelemicin 487-12-7 C12H16O3 208.257 —— 3-[(3,4,5-Trimethoxyphenyl)methyl]-1,2,4-trioxolane 1373139-34-4 C12H16O6 256.255 —— 4-methoxy-6-methylbenzo[d][1,3]dioxole 6443-70-5 C9H10O3 166.177 (2E)-3-(3,4,5-三甲氧基苯基)丙烯醛 3,4,5-trimethoxycinnamaldehyde 71277-13-9 C12H14O4 222.241 3-(3,4,5-三甲氧基苯基)丙-2-烯醛 3,4,5-trimethoxycinnamaldehyde 34346-90-2 C12H14O4 222.241 1,3-二甲氧基-5-丙基苯 1,3-dimethoxy-5-propylbenzene 41395-10-2 C11H16O2 180.247 —— (R)-3-(3,4,5-trimethoxyphenyl)-2-O-(4-allyl-2,6-dimethoxyphenyl)-1,2-propanediol —— C23H30O7 418.487 3,4,5-三甲氧基苯甲酸 Eudesmic acid 118-41-2 C10H12O5 212.202 肉豆蔻醛 3-Methoxy-4,5-methylenedioxybenzaldehyde 5780-07-4 C9H8O4 180.16 —— isomyristicin 487-62-7 C11H12O3 192.214 —— (S)-3-(3,4,5-trimethoxyphenyl)-1-O-benzoyl-1,2-propanediol 638168-43-1 C19H22O6 346.38 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:SYNTHESIS OF THE TRIMETHOXYPHENYLPROPENES摘要:不可用。DOI:10.1139/v65-478
-
作为产物:描述:参考文献:名称:新木脂素 Rhaphidecursinol A 和 Virolongin B 的首次对映选择性合成摘要:首次报道了新木脂素 Rhaphidecursinol A 和 Virolongin B 的对映选择性合成。合成中的关键反应是Sharpless不对称二羟基化和Mitsunobu反应。Rhaphidecursinol A 的绝对构型得到确认。DOI:10.1002/jccs.200400144
文献信息
-
NiH‐Catalyzed Migratory Defluorinative Olefin Cross‐Coupling: Trifluoromethyl‐Substituted Alkenes as Acceptor Olefins to Form <i>gem</i> ‐Difluoroalkenes作者:Fenglin Chen、Xianfeng Xu、Yuli He、Genping Huang、Shaolin ZhuDOI:10.1002/anie.201915840日期:2020.3.23a NiH-catalyzed migratory defluorinative coupling between two electronically differentiated olefins. A broad range of unactivated donor olefins can be joined directly to acceptor olefins containing an electron-deficient trifluoromethyl substituent in both intra- and intermolecular fashion to form gem-difluoroalkenes. This migratory coupling shows both site- and chemoselectivity under mild conditions
-
A Facile, Convenient, and Green Route to (E)-Propenylbenzene Flavors and Fragrances by Alkene Isomerization作者:Douglas Grotjahn、Casey Larsen、Erik Paulson、Gulin ErdoganDOI:10.1055/s-0035-1560205日期:——(E)-Propenylbenzene flavors and fragrances can be made andisolated in high yield and selectivity by using bifunctional catalyst 1, andthe heterogenized analogues. Multigram-scale reactions can be performed neat and the products isolated either by distillation, using homogeneous catalyst 1 (0.1-0.5 mol%, r.t., 10-45 min), or by decantation from heterogeneous catalysts PS-1 or PSL-1 (0.5 mol%, 70 degrees C, 24 h; catalyst separation and re-use shown for 3-4 cycles; 10 cycles using distilled eugenol feedstock). Both purified starting materials and essential oil feedstocks could be used. Z Isomers were present at very low levels (from 0.4% to less than 0.1%) in the products.
-
Palladium-Catalyzed Allylic C–H Oxidative Annulation for Assembly of Functionalized 2-Substituted Quinoline Derivatives作者:Chunsheng Li、Jianxiao Li、Yanni An、Jianwen Peng、Wanqing Wu、Huanfeng JiangDOI:10.1021/acs.joc.6b01909日期:2016.12.16An efficient and practical palladium-catalyzed aerobic oxidative approach to afford functionalized 2-substituted quinolines in moderate to good yields from readily available allylbenzenes with aniline is developed. The present annulation process has high functional-group tolerance and high atom economy, making it a valuable and practical method in synthetic and medicinal chemistry. Moreover, this transformation
-
Comparing the Stereoselective Biooxidation of Cyclobutanones by Recombinant Strains Expressing Bacterial Baeyer–Villiger Monooxygenases作者:Florian Rudroff、Joanna Rydz、Freek H. Ogink、Michael Fink、Marko D. MihovilovicDOI:10.1002/adsc.200700072日期:2007.6.4cyclobutanone structural motif was investigated using a collection of eight monooxygenases of different bacterial origin. This platform of enzymes is able to perform stereoselective biotransformations on an array of structurally diverse substrates. With several ketone precursors, biooxidations yielded enantiocomplementary butyrolactones as key intermediates for the synthesis of natural products and bioactive compounds
-
Cobalt-Catalyzed Trifluoromethylation–Peroxidation of Unactivated Alkenes with Sodium Trifluoromethanesulfinate and Hydroperoxide作者:Hong-Yu Zhang、Chao Ge、Jiquan Zhao、Yuecheng ZhangDOI:10.1021/acs.orglett.7b02353日期:2017.10.6herein is an unprecedented cobalt-catalyzed trifluoromethylation–peroxidation of unactivated alkenes. In this process the hydroperoxide acts as a radical initiator as well as a coupling partner. The cheap and readily available sodium trifluoromethanesulfinate serves as the CF3 source in the reaction. Various alkenes are transformed into vicinal trifluoromethyl-peroxide compounds in moderate to good yields
表征谱图
-
氢谱1HNMR
-
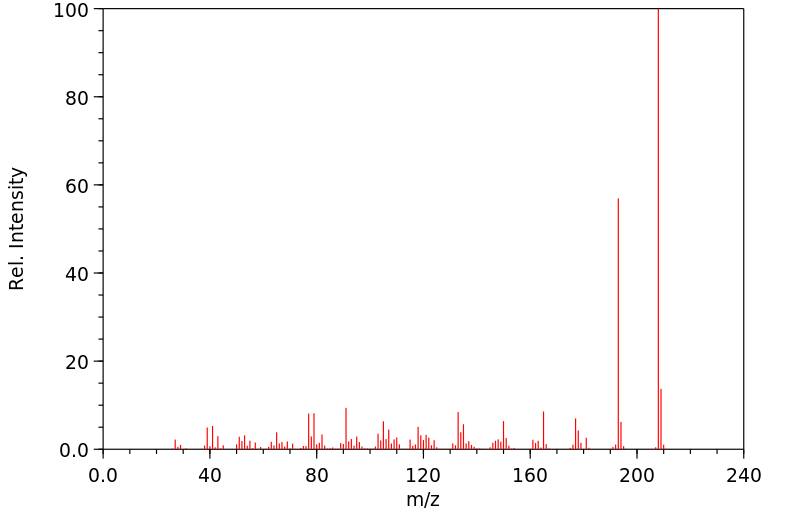
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯