十二氢苯并菲 | 1610-39-5
中文名称
十二氢苯并菲
中文别名
十二氢三亚苯
英文名称
1,2,3,4,5,6,7,8,9,10,11,12-dodecahydrotriphenylene
英文别名
dodecahydrotriphenylene
CAS
1610-39-5
化学式
C18H24
mdl
——
分子量
240.389
InChiKey
ODHYDPYRIQKHCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:231-233 °C (lit.)
-
沸点:318.11°C (rough estimate)
-
密度:0.9356 (estimate)
-
溶解度:可溶于己烷
-
保留指数:386.36
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:18
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2902909090
-
危险品运输编号:UN 2811 6.1/PG 3
-
储存条件:密封储存,存放在阴凉、干燥且通风良好的仓库中,并远离不相容的物质。
SDS
| Name: | Dodecahydrotriphenylene 99% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 1610-39-5 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1610-39-5 | Dodecahydrotriphenylene | 99 | 216-550-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1610-39-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 231.00 - 233.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C18H24
Molecular Weight: 240.39
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1610-39-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Dodecahydrotriphenylene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1610-39-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1610-39-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1610-39-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4,5,6,7,8-八氢菲 1,2,3,4,5,6,7,8-Octahydrophenanthrene 5325-97-3 C14H18 186.297 对称八氢蒽 1,2,3,4,5,6,7,8-octahydroanthracene 1079-71-6 C14H18 186.297 4-(5,6,7,8-四氢萘-2-基)丁酸 4-(5,6,7,8-tetrahydronaphthalen-2-yl)butanoic acid 782-27-4 C14H18O2 218.296 1,2,3,4-四氢萘 tetralin 119-64-2 C10H12 132.205 2-氯-1-(5,6,7,8-四氢萘-2-基)乙酮 1,2,3,4-Tetrahydro-7-chloracetylnaphthalin 5803-67-8 C12H13ClO 208.688 5,6,7,8-四氢-gamma-氧萘-2-丁酸 4-oxo-4-(5,6,7,8-tetrahydronaphthalen-2-yl)butanoic acid 785-17-1 C14H16O3 232.279 —— 3,4,5,6,7,8-hexahydro-1(2H)-anthracenone 5440-71-1 C14H16O 200.28 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,4,5,8,9,12-hexabromododecahydrotriphenylene —— C18H18Br6 713.765 —— 1,4,5,8,9,12-hexabromododecahydrotriphenylene —— C18H18Br6 713.765
反应信息
-
作为反应物:参考文献:名称:Mannich, Chemische Berichte, 1907, vol. 40, p. 160摘要:DOI:
-
作为产物:参考文献:名称:碳纳米管支撑的双金属Pt-Rh纳米粒子的轻松声化学合成,用于芳烃的室温加氢†摘要:双金属Pt–Rh纳米粒子可以使用简单的一步声化学方法均匀地沉积在羧酸盐官能化的多壁碳纳米管(MWNT)的表面上。相对于用于纯净的多环芳烃(PAHs)催化加氢的单个Pt或Rh金属纳米颗粒,双金属纳米颗粒催化剂表现出强大的协同作用。苯和烷基苯。在室温下,使用双金属Pt–Rh / MWNTs催化剂可实现PAHs完全环饱和。这一一步合成技术提供了一种简单而快速的方法,可用于制备高活性且可回收的CNT负载的单金属和双金属纳米催化剂,用于低温加氢反应。DOI:10.1039/c1nj20028c
-
作为试剂:参考文献:名称:亚胺插入后期金属-碳键形成稳定的酰胺配合物摘要:制备了含有不稳定配位体的芳基铑 (I) 配合物,并研究了其对芳基亚胺的反应性。芳基铑 (I) 络合物 (DPPE)Rh(C5H5N)(p-tol), 2 以 65% 的产率从 [(DPPE)Rh(mu-Cl)]2、吡啶和对甲苯基锂中分离出来。2与醛亚胺(p-tol)CH=N(C6H4-p-CO2Me)(3a-Tol)的反应以88%的分离产率得到Rh酰胺插入产物4。4 的固态结构是通过单晶 X 射线衍射确定的。2 与电子中性和富电子醛亚胺 (Ph)CH=NPh (3b) 和 (p-tol)CH=N(C6H4-p-OMe) (3c) 的反应似乎也涉及插入,但由这些插入形成的酰氨基复合物不稳定。因此,2 与 3b 反应,然后加入 Et3NHCl,得到胺和酮亚胺产物 (Ph)(p-tol)CH-NHPh, 5,和 (p-tol)(Ph)C=N(Ph), 6、收益率分别为 25% 和 50%。几行数DOI:10.1021/ja038819a
文献信息
-
Strong Lewis Acids of Air-Stable Metallocene Bis(perfluorooctanesulfonate)s as High-Efficiency Catalysts for Carbonyl-Group Transformation Reactions作者:Renhua Qiu、Xinhua Xu、Lifeng Peng、Yalei Zhao、Ningbo Li、Shuangfeng YinDOI:10.1002/chem.201103874日期:2012.5.14that 2 a and 2 b were thermally stable at 300 and 180 °C, respectively. These complexes exhibited unusually high solubility in polar organic solvents. Conductivity measurement showed that the complexes (2 a and 2 b) were ionic dissociation in CH3CN solution. X‐ray analysis result confirmed 2 a⋅3 H2O⋅THF was a cationic organometallic Lewis acid. UV/Vis spectra showed a significant red shift due to the强路易斯酸空气中稳定的金属茂双(全氟辛烷磺酸盐)S [M(CP)2 ] [OSO 2 C ^ 8 ˚F 17 ] 2 ⋅ Ñ ħ 2 O⋅THF(M = Zr的(2 ⋅3ħ 2 O⋅THF ),M =的Ti(图2b ⋅2ħ 2 O⋅THF))由[M(CP)的反应合成2 ]氯2(M = Zr的(1),M =的Ti(图1b)),与n BuLi和C 8 F 17 SO 3 H(2当量)或与C 8 F 17 SO 3Ag(2当量)。这些配合物的水合物数(n)是可变的,取决于条件从0变为4。与众所周知的茂金属三氟甲磺酸盐相反,这些络合物在露天环境中一年没有变化。热重-差示扫描量热(TG-DSC)分析表明,2和图2b分别为300和180℃,是热稳定的。这些配合物在极性有机溶剂中显示出异常高的溶解度。电导率测量表明,络合物(2a和2b)在CH 3 CN溶液中呈离子离解。X射线分析结果确认为2 a⋅3H
-
H<sub>6</sub>P<sub>2</sub>W<sub>18</sub>O<sub>62</sub>/Nanoclinoptilolite as an efficient nanohybrid catalyst in the cyclotrimerization of aryl methyl ketones under solvent-free conditions作者:R. Tayebee、M. JarrahiDOI:10.1039/c5ra01344e日期:——wide range of alkyl, aryl, and cyclic ketones. The nanocatalyst was prepared via immobilization of Wells–Dawson heteropolyacid H6P2W18O62 (HPA) on the surface of nanoclinoptilolite (NCP). The nanohybrid material was easily recovered and reused successfully at least seven times without significant loss of catalytic activity. XRD, SEM, UV-Vis, MS-ICP, DTA, and FT-IR studies confirmed that the heteropolyacid制备了一种新型的纳米杂化材料H 6 P 2 W 18 O 62 /纳米斜发沸石,并作为一种高效且可重复使用的催化剂,在不同的苯乙酮的温和单锅缩合反应中使用。本方法学的操作简便,易于后处理,具有成本效益和无溶剂的性质,可从多种烷基,芳基和环状物中得到所需的1,3,5-三芳基苯酮。通过固定Wells-Dawson杂多酸H 6 P 2 W 18 O 62制备纳米催化剂(HPA)在纳米斜发沸石(NCP)的表面上。纳米混合材料易于回收并成功重复使用至少七次,而催化活性没有明显损失。XRD,SEM,UV-Vis,MS-ICP,DTA和FT-IR研究证实,杂多酸很好地分散在NCP的表面上。开发的该方案是一种利用生态友好且高度可重复使用的天然纳米催化剂合成1,3,5-三芳基苯的安全便捷的替代方法。此外,水是唯一的副产物,这使得本方法学对环境无害。
-
Novel Method for the Synthesis of 1,3,5‐Triarylbenzenes from Ketones作者:Xiaobi Jing、Feng Xu、Qihua Zhu、Xinfeng Ren、Chaoguo Yan、Li Wang、Jinrong WangDOI:10.1080/00397910500283370日期:2005.12.1Abstract Treatment of aryl ketones with p‐toluenesulfinic acid and a catalytic amount of tin tetrachloride anhydrous (5%) in 1‐pentanol gives good yields of 1,3,5‐triarylbenzenes.
-
A new method for the preparation of 1,3,5-triarylbenzenes catalyzed by nanoclinoptilolite/HDTMA作者:R. Tayebee、M. Jarrahi、B. Maleki、M. Kargar Razi、Z. B. Mokhtari、S. M. BaghbanianDOI:10.1039/c4ra11216d日期:——
An efficient method for the preparation of 1,3,5-triarylbenzenes. Natural surface-modified NCP as a new and reusable catalyst. Using solvent-free conditions.
一种高效的制备1,3,5-三芳基苯的方法。天然表面改性的NCP作为一种新型可重复使用的催化剂。采用无溶剂条件。 -
C2 molecule: formation from bromoacetylene and reactions with cyclohexene or 2,3-dimethyl-2-butene作者:Nicolas Galy、Henri Doucet、Maurice SantelliDOI:10.1016/j.tetlet.2009.11.113日期:2010.1The C2 molecule (1,2-ethynediyl) has been prepared by dehydrohalogenation of 1,2-dibromoethylene with an excess of potassium tert-butoxide in 2,3-dimethyl-2-butene as the solvent and the reagent. The major products of this reaction were 2,3-dimethylbut-3-en-2-ol and dibromoacetylene.
表征谱图
-
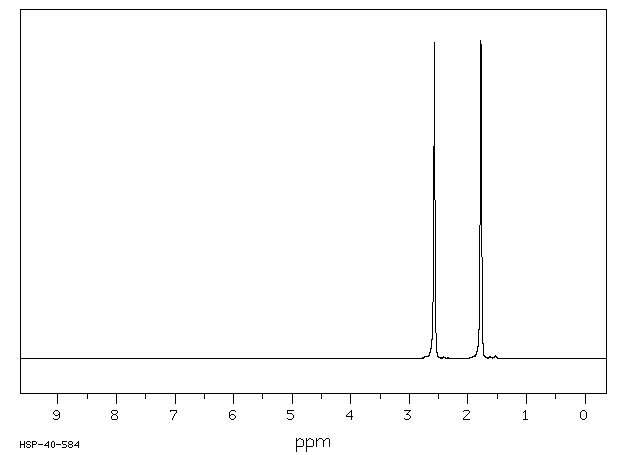
氢谱1HNMR
-
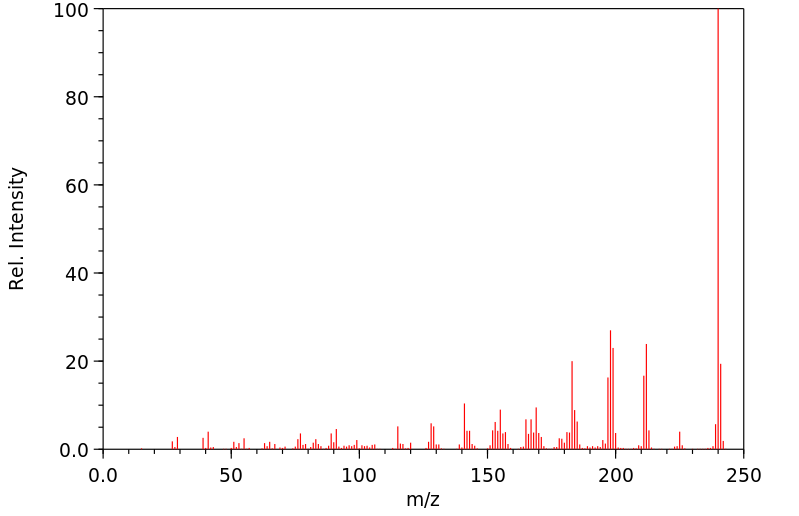
质谱MS
-
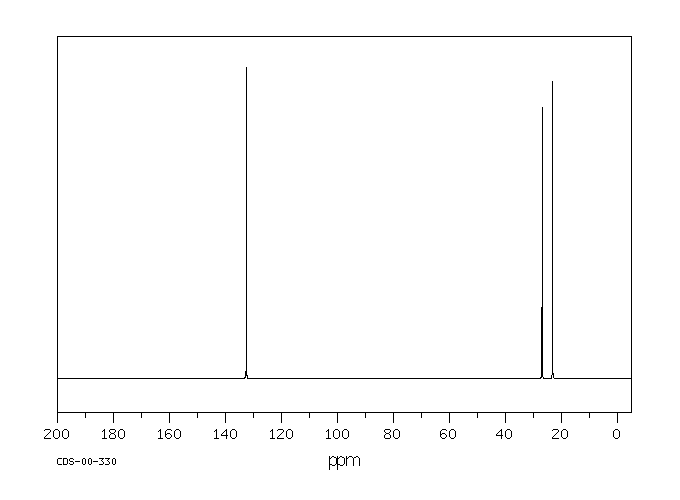
碳谱13CNMR
-
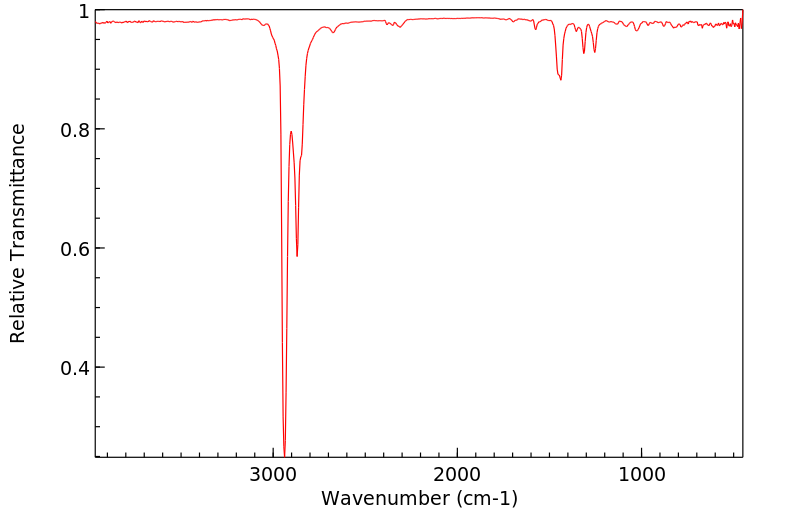
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩