溴代环辛烷 | 1556-09-8
中文名称
溴代环辛烷
中文别名
——
英文名称
cyclooctyl bromide
英文别名
bromocyclooctane
CAS
1556-09-8
化学式
C8H15Br
mdl
——
分子量
191.111
InChiKey
KFKLBMQLKLKHLU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:102-103°C 18mm
-
密度:1.282 g/cm3
-
闪点:102-103°C/18mm
-
保留指数:1258
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知的危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
海关编码:2903890090
-
储存条件:请将贮藏器密封保存在阴凉干燥处,并确保工作环境有良好的通风或排气设施。
SDS
反应信息
-
作为反应物:参考文献:名称:Selective reductions. 31. Lithium triethylborohydride as an exceptionally powerful nucleophile. A new and remarkably rapid methodology for the hydrogenolysis of alkyl halides under mild conditions摘要:DOI:10.1021/jo00166a031
-
作为产物:参考文献:名称:未活化的烷基 sp3 C—H 键与碘 (III) 的高效光解卤化和氧化摘要:据报道,使用碘(III)作为可见光或KBr下可行的脱氢剂,对未活化的烷基sp 3 C—H键进行无金属、绿色和可持续的官能化,并且可以构建烷基氯、溴化物、醇和酮通过添加不同的偶联剂。采用廉价、安全的二乙酸碘苯形成自由基,高效激活烷基sp 3 C—H键,通过添加相应的偶联剂可以构建不同的烷基化产物。DOI:10.1002/cjoc.202300544
-
作为试剂:描述:RhIII(5,10,15,20-tetrakis(2,4,6-trimethylphenyl)porphyrinato)Cl 、 sodium tetrahydroborate 在 sodium hydroxide 、 溴代环辛烷 作用下, 以 乙醇 、 水 为溶剂, 反应 1.25h, 以89%的产率得到Rh(tetrakismesitylporphyrinato)H参考文献:名称:Metalloradical-Catalyzed Selective 1,2-Rh-H Insertion into the Aliphatic Carbon–Carbon Bond of Cyclooctane摘要:The selective aliphatic carboncarbon activation of cyclo-octane (c-octane) was achieved via the Rh-II(ttp)-catalyzed 1,2-addition of Rh(ttp)H to give Rh(ttp)(n-octyl) (ttp = tetratolylporphyrinato dianion) in good yield under mild reaction conditions. This mechanism is further supported by DFT calculations. The reaction worked only with the sterically accessible Rh(ttp) porphyrin complex but not with the bulky Rh(tmp) system (tmp = tetrakismesitylporphyrinato dianion), thus showing the highly steric sensitivity of carboncarbon bond activation by transition metal complexes.DOI:10.1021/acs.organomet.5b00183
文献信息
-
Efficient C(sp3alkyl)–SCF3 bond formations via copper-mediated trifluoromethylthiolation of alkyl halides作者:Quanfu Lin、Li Chen、Yangjie Huang、Mingguang Rong、Yaofeng Yuan、Zhiqiang WengDOI:10.1039/c4ob00403e日期:——A general and convenient copper-mediated trifluoromethylthiolation of primary and secondary alkyl halides was described. Variation of the solvent, additives and time allowed optimization of the reaction. A wide range of alkyl halides were explored to give a set of alkyl trifluoromethyl thioethers in moderate to excellent yields. A variety of functional groups, including ethers, thioether, esters, nitriles, amides, and ketal groups, were well tolerated in the electrophilic partner.
-
Fischer Indole Synthesis with Organozinc Reagents作者:Benjamin A. Haag、Zhi-Guang Zhang、Jin-Shan Li、Paul KnochelDOI:10.1002/anie.201005319日期:2010.12.3Updated classic: Primary and secondary alkylzinc reagents add to various aryldiazonium salts leading regioselectively to polyfunctional indoles by means of a [3,3]‐sigmatropic shift and subsequent aromatization. This organometallic variation of the Fischer indole synthesis tolerates a wide range of functional groups and displays absolute regioselectivity.
-
A Photoirradiative Phase-Vanishing Method: Efficient Generation of HBr from Alkanes and Molecular Bromine and Its Use for Subsequent Radical Addition to Terminal Alkenes作者:Hiroshi Matsubara、Ilhyong Ryu、Masaaki Tsukida、Daisuke Ishihara、Kenji KuniyoshiDOI:10.1055/s-0030-1258482日期:2010.8phase-vanishing (PV) system comprised of an alkane, perfluorohexanes, and bromine was successfully combined by photoirradiation to efficiently generate hydrogen bromide, which underwent radical addition with 1-alkenes in the hydrocarbon layer to afford terminal bromides in high yields.
-
Highly selective halogenation of unactivated C(sp<sup>3</sup>)–H with NaX under co-catalysis of visible light and Ag@AgX作者:Shouxin Liu、Qi Zhang、Xia Tian、Shiming Fan、Jing Huang、Andrew WhitingDOI:10.1039/c8gc02628a日期:——The direct selective halogenation of unactivated C(sp3)–H bonds into C-halogen bonds was achieved using a nano Ag/AgCl catalyst at RT under visible light or LED irradiation in the presence of an aqueous solution of NaX/HX as a halide source, in air. The halogenation of hydrocarbons provided mono-halide substituted products with 95% selectivity and yields higher than 90%, with the chlorination of toluene
-
The Synthesis of Chiral Allyl Carbamates via Merger of Photoredox and Nickel Catalysis作者:Mateusz Garbacz、Sebastian SteckoDOI:10.1002/adsc.202000404日期:2020.8.4A mild, and versatile, organophotoredox/Ni‐mediated protocol was developed for the direct preparation of diverse, enantioenriched allyl carbamates. The reported approach represents a significant departure from classical step‐by‐step synthesis of allyl carbamates. This dual photoredox/Ni based strategy offers unrivalled capacity for convergent unification of readily available alkyl halides and chiral开发了一种温和且通用的有机光氧化还原/ Ni介导的方案,用于直接制备各种对映体富集的烯丙基氨基甲酸酯。报道的方法代表了烯丙基氨基甲酸酯经典一步一步合成的重大偏离。这种基于光氧化还原/镍的双重策略提供了无与伦比的能力,能够以较高的化学选择性和效率将衍生自1-溴代烯烃3-醇的烷基卤化物和手性氨基甲酸酯聚合统一。报道的光氧化还原/镍催化的交叉偶联反应不仅限于氨基甲酸酯,还包括其他O。衍生物,例如酯,醚,缩醛,碳酸酯或甲硅烷基醚。为了证明所报道方案的实用性,将所得的氨基甲酸烯丙酯通过对映体特异性方式的σ重排反应转化为功能化的非外消旋烯丙胺。该方法允许通过简单控制烯丙基氨基甲酸酯的双键的几何形状来合成对映异构烯丙基胺。
表征谱图
-
氢谱1HNMR
-
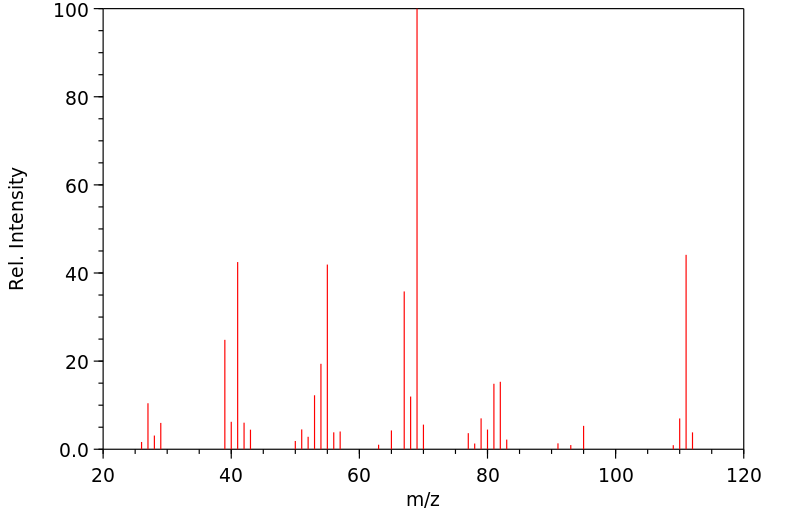
质谱MS
-
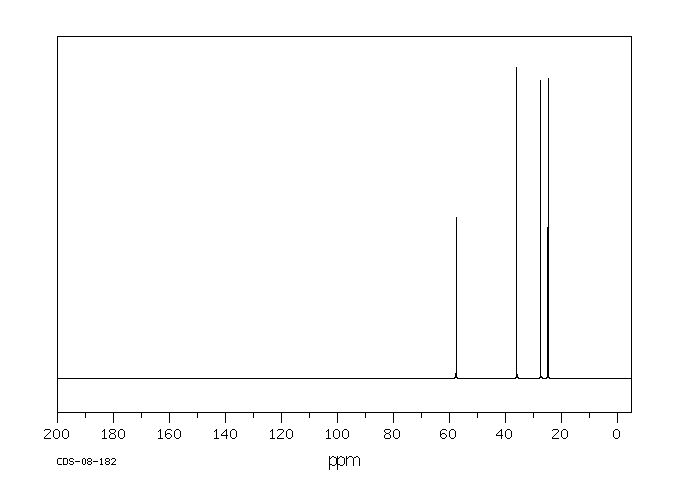
碳谱13CNMR
-
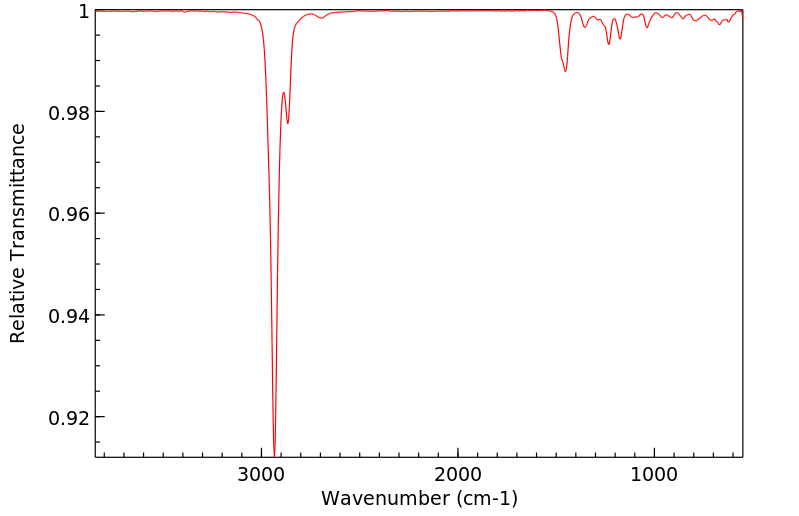
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4