辛基 己酸酯 | 4887-30-3
物质功能分类
中文名称
辛基 己酸酯
中文别名
辛基己酸酯
英文名称
n-octyl hexanoate
英文别名
octyl hexanoate;hexanoic acid octyl ester;Hexansaeure-octylester;Capronsaeure-octylester
CAS
4887-30-3
化学式
C14H28O2
mdl
MFCD00048941
分子量
228.375
InChiKey
CMNMHJVRZHGAAK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-28°C
-
沸点:275.25°C
-
密度:0.8603
-
LogP:5.880 (est)
-
保留指数:1570;1565;1572;1567;1567;1575
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:16
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.928
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Spontaneous Generation of Optical Activity in Urea Inclusion Compounds vis-a-vis Current Theories摘要:在脲通道中加入某些 2-甲基己酸烷基酯的基础上,我们设计了一种新的方法来检验光学活性起源的最新理论。DOI:10.1039/a707831e
-
作为产物:描述:5-Oxo-hexanoic acid octyl ester 在 sodium cyanoborohydride 、 对甲苯磺酸 、 对甲苯磺酰肼 作用下, 以 环丁砜 、 N,N-二甲基甲酰胺 为溶剂, 生成 辛基 己酸酯参考文献:名称:Selective deoxygenation of ketones and aldehydes including hindered systems with sodium cyanoborohydride摘要:DOI:10.1021/ja00792a033
文献信息
-
Recyclable Hypervalent Iodine(III) Reagent Iodosodilactone as an Efficient Coupling Reagent for Direct Esterification, Amidation, and Peptide Coupling作者:Jun Tian、Wen-Chao Gao、Dong-Mei Zhou、Chi ZhangDOI:10.1021/ol301085v日期:2012.6.15hypervalent iodine(III) reagent plays a novel role as an efficient coupling reagent to promote the direct condensation between carboxylic acids and alcohols or amines to provide esters, macrocyclic lactones, amides, as well as peptides without racemization. The regeneration of iodosodilactone (1) can also be readily achieved. The intermediate acyloxyphosphonium ion C from the activation of a carboxylic acid
-
Acylation of Alcohols and Amines with Vinyl Acetates Catalyzed by Cp*<sub>2</sub>Sm(thf)<sub>2</sub>作者:Yasutaka Ishii、Mitsuhiro Takeno、Yumi Kawasaki、Akifumi Muromachi、Yutaka Nishiyama、Satoshi SakaguchiDOI:10.1021/jo952168m日期:1996.1.1Cp(2)Sm(thf)(2) was found to be an efficient catalyst for the acylation of alcohols and amines with esters under mild conditions. In the present acylation, vinyl and isopropenyl acetates served as good acylating agents. Thus, a variety of alcohols and amines underwent acylation with vinyl and isopropenyl acetates in the presence of Cp(2)Sm(thf)(2) to give the corresponding esters and amides in good
-
Novel Brønsted Acidic Ionic Liquids and Their Use as Dual Solvent−Catalysts作者:Amanda C. Cole、Jessica L. Jensen、Ioanna Ntai、Kim Loan T. Tran、Kristin J. Weaver、David C. Forbes、James H. DavisDOI:10.1021/ja026290w日期:2002.5.1The reaction of triphenylphosphine or N-butylimidazole with cyclic sultones gives zwitterions that are subsequently converted into ionic liquids by reaction with trifluoromethane sulfonic acid or p-toluenesulfonic acid. The resulting ionic liquids have cations to which are tethered alkane sulfonic acid groups. These Brønsted acidic ionic liquids are useful solvent/catalysts for several organic reactions
-
[EN] PROCESSES FOR PRODUCING CARBOXYLIC ACIDS<br/>[FR] PROCÉDÉS DE PRODUCTION D'ACIDES CARBOXYLIQUES申请人:EASTMAN CHEM CO公开号:WO2020205348A1公开(公告)日:2020-10-08Processes are disclosed for preparing carboxylic acids from organic esters, the processes comprising contacting an ester with water in the presence of an acid catalyst and a homogenizing solvent at conditions effective to form a carboxylic acid. The homogenizing solvent is present in an amount sufficient to form a single-phase reaction mixture comprising the ester, water, and homogenizing solvent. The homogenizing solvent may be selected from acetonitrile, dimethyl sulfoxide, and 1,4-dioxane.
-
Aerobic Self‐Esterification of Alcohols Assisted by Mesoporous Manganese and Cobalt Oxide作者:Ehsan Moharreri、Sourav Biswas、Bahareh Deljoo、David Kriz、Seyoung Lim、Sarah Elliott、Shanka Dissanayake、Marina Dabaghian、Mark Aindow、Steven L. SuibDOI:10.1002/cctc.201900704日期:2019.8.7alcohols catalyzed by mesoporous metal oxides (manganese and cobalt oxides) is reported under base and solvent free conditions. For a range of aliphatic alcohols, up to 90 % conversions to esters was achieved. The catalytic reaction is likewise applicable to neat aldehydes as substrates with yields of up to 86 %. High pressure batch reaction for ethanol to ethyl acetate led to 22 % yield. Isotope labeling
表征谱图
-
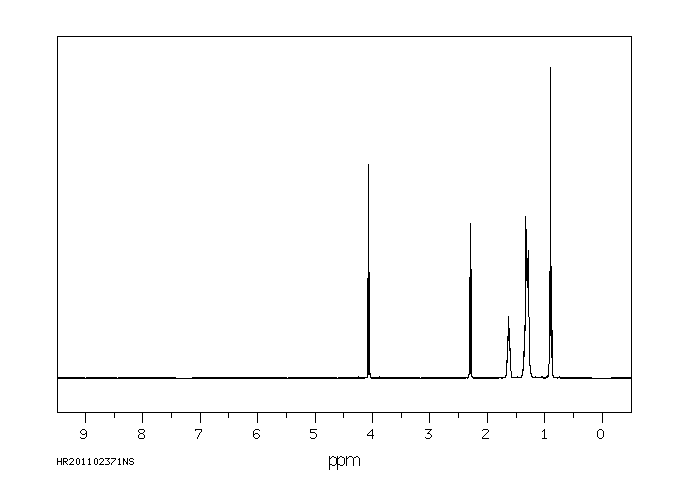
氢谱1HNMR
-
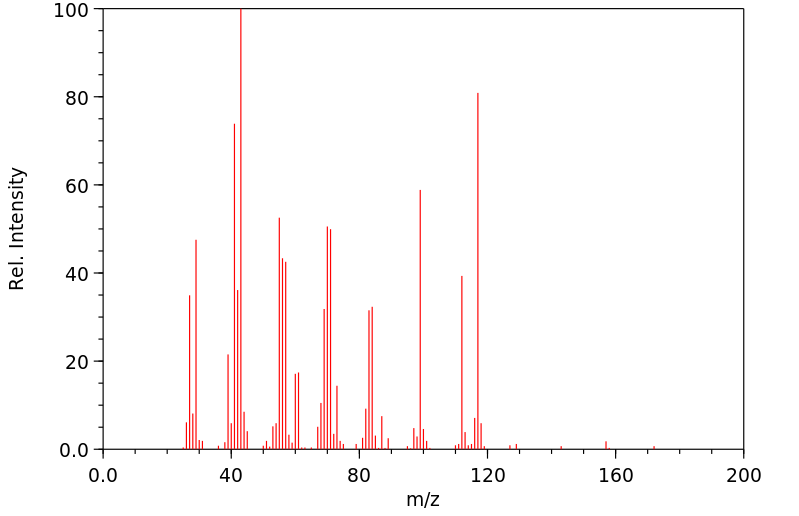
质谱MS
-
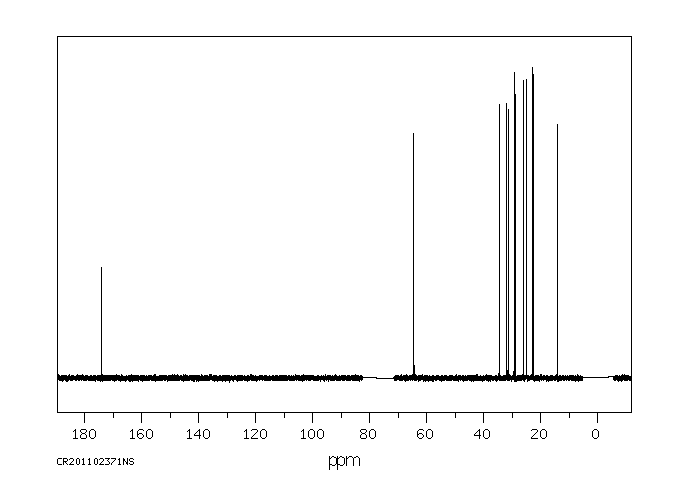
碳谱13CNMR
-
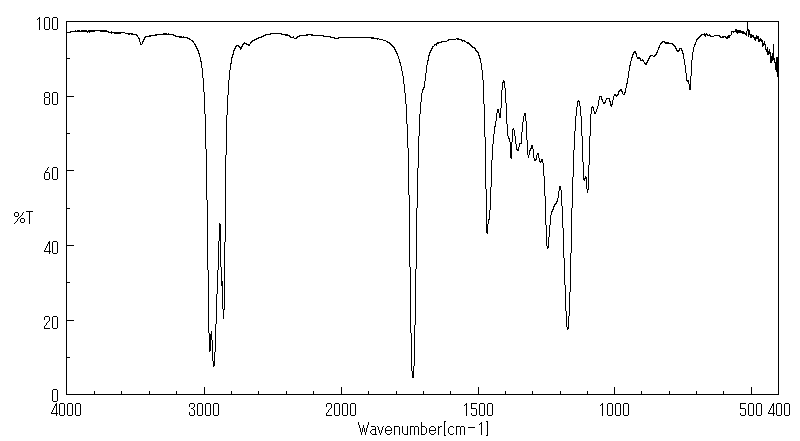
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯