2-邻苯二甲酰亚胺基乙酸甲酯 | 23244-58-8
中文名称
2-邻苯二甲酰亚胺基乙酸甲酯
中文别名
邻苯二甲酰亚胺甘氨酸甲脂;2-邻苯二甲酰亚氨基乙酸甲酯
英文名称
N-phthaloylglycine methyl ester
英文别名
methyl 2-(1,3-dioxoisoindolin-2-yl)acetate;N-Phthaloyl-glycin-methylester;phthaloylglycine methyl ester;methyl N-phthaloylglycinate;Phth-Gly-OMe;methyl 2-(1,3-dioxoisoindol-2-yl)acetate
CAS
23244-58-8
化学式
C11H9NO4
mdl
MFCD00158668
分子量
219.197
InChiKey
JINKRJDUNDRREU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:113 °C
-
沸点:345.7±25.0 °C(Predicted)
-
密度:1.368±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.181
-
拓扑面积:63.7
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2925190090
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P273,P301+P310,P305+P351+P338
-
危险品运输编号:2811
-
危险性描述:H301,H410
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Methyl 2-phthalimidoacetate
Synonyms: Methyl 2-(1,3-dioxoisoindol-2-yl)acetate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 2-phthalimidoacetate
CAS number: 23244-58-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H9NO4
Molecular weight: 219.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Methyl 2-phthalimidoacetate
Synonyms: Methyl 2-(1,3-dioxoisoindol-2-yl)acetate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 2-phthalimidoacetate
CAS number: 23244-58-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H9NO4
Molecular weight: 219.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 邻苯二甲酰甘氨酸 N-phthaloylglycine 4702-13-0 C10H7NO4 205.17 N-邻苯二甲酰甘氨酰氯 N-phthaloylglycine chloride 6780-38-7 C10H6ClNO3 223.616 N-烯丙基邻苯二甲酰亚胺 3-phthalimido-1-propene 5428-09-1 C11H9NO2 187.198 N-溴甲基邻苯二甲酰亚胺 N-(bromomethyl)phtalimide 5332-26-3 C9H6BrNO2 240.056 N-氯甲基邻苯二甲酰亚胺 N-(chloromethyl)phthalimide 17564-64-6 C9H6ClNO2 195.605 2-(1,3-二氢异吲哚-2-基)乙酸甲酯 methyl 2-(1,3-dihydroisoindol-2-yl)acetate 363165-74-6 C11H13NO2 191.23 邻苯二甲酸亚胺 phthalimide 85-41-6 C8H5NO2 147.133 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-乙氧基羰基甲基邻苯二甲酰亚胺 phthalimidoacetic acid ethyl ester 6974-10-3 C12H11NO4 233.224 —— N,N-phthaloyl-glycine-propyl ester 40531-88-2 C13H13NO4 247.251 邻苯二甲酰甘氨酸 N-phthaloylglycine 4702-13-0 C10H7NO4 205.17 —— butyl 2-(1,3-dioxoisoindolin-2-yl)acetate 95750-89-3 C14H15NO4 261.277 —— 3-oxo-2-phthalimido-propionic acid methyl ester 41400-42-4 C12H9NO5 247.207 —— 2-phthalimidoacetamide 4935-96-0 C10H8N2O3 204.185 (1-羟基-3-氧代-1,3-二氢-异吲哚-2-基)-乙酸甲酯 methyl 2-(1-hydroxy-3-oxoisoindolin-2-yl)acetate 100166-98-1 C11H11NO4 221.213 —— nijmegen 1 159155-03-0 C17H13NO7 343.293 —— (Z)-2-(2-methoxy-2-((trimethylsilyl)oxy)vinyl)isoindoline-1,3-dione 1255468-00-8 C14H17NO4Si 291.379 —— 2-(1,3-dioxo-1,3-dihydro-(2H)-isoindole-2-ylmethyl)-5-amino-1,3,4-thiadiazole —— C11H8N4O2S 260.276
反应信息
-
作为反应物:描述:2-邻苯二甲酰亚胺基乙酸甲酯 在 N-甲基吡咯烷酮 、 [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 、 水 、 potassium carbonate 作用下, 反应 24.0h, 以50%的产率得到马尿酸乙酯参考文献:名称:揭露酰胺:N取代邻苯二甲酰亚胺衍生物的钌催化原羰基化摘要:使用钌催化剂,以高收率和较短的反应时间实现了单步操作中各种合成吸引力的邻苯二甲酰亚胺前所未有的转化为酰胺。机理研究表明,独特的均质途径涉及五元环的打开和CO 2的释放,其中水是质子的来源。DOI:10.1021/acs.orglett.7b03278
-
作为产物:描述:3-chloro-3-(dimethoxyphosphoryl)isobenzofuran-1(3H)-one 在 氯化亚砜 、 N,N-二异丙基乙胺 作用下, 以 水 、 乙腈 为溶剂, 反应 4.5h, 生成 2-邻苯二甲酰亚胺基乙酸甲酯参考文献:名称:环境自由基氧化剂NO 3 •对脂族氨基酸,二肽和三肽的氧化损伤:动力学和计算研究揭示酰胺键的作用摘要:动力学和计算数据表明,重要的环境自由基氧化剂NO 3 •在与脂肪族氨基酸以及二肽和三肽的反应中具有复杂的行为,这表明对肽主链中酰胺N–H键的攻击是高度可行的途径,这是通过质子耦合电子转移(PCET)机理进行的,在乙腈中的速率系数约为1×10 6 M –1 s –1。确定了类似的速率系数,用于确定侧链中α-碳和叔C–H键的夺氢作用。获得的NO 3 •反应速率系数带有脂族二肽和三肽的化合物表明,攻击在每个氨基酸残基的所有这些位点发生,这使得脂族肽序列极易受到NO 3 •诱导的氧化损伤的影响。NO 3 •与脂肪族肽中侧链的反应没有发现酰胺邻基效应的证据,酰胺邻基效应先前已发现可促进自由基诱导的苯丙氨酸侧链破坏。DOI:10.1021/acs.joc.8b03224
文献信息
-
Oxidative damage of proline residues by nitrate radicals (NO<sub>3</sub>˙): a kinetic and product study作者:Joses G. Nathanael、Jonathan M. White、Annika Richter、Madison R. Nuske、Uta WilleDOI:10.1039/d0ob01337d日期:——indicating that NO3˙-induced oxidation of amide bonds proceeds through initial formation of a charge transfer complex. Furthermore, the rate of oxidative damage of proline and N-methyl glycine is significantly influenced by its position in a peptide. Thus, neighbouring peptide bonds, particularly in the N-direction, reduce the electron density at the tertiary amide, which slows down the rate of ET by up叔酰胺,例如N-酰化脯氨酸或N-甲基甘氨酸残基,与硝酸根 (NO 3 ˙) 快速反应,在乙腈中的绝对速率系数范围为 4-7 × 10 8 M -1 s -1 。主要途径通过氮处的氧化电子转移 (ET) 进行,而在这些条件下,夺氢只是次要因素。然而,酰胺的空间位阻,例如α-碳上的烷基侧链,使速率系数降低高达 75%,表明 NO 3˙ 诱导的酰胺键氧化通过电荷转移复合物的初始形成进行。此外,脯氨酸和N-甲基甘氨酸的氧化损伤率显着受其在肽中的位置的影响。因此,相邻的肽键,特别是在N方向上,会降低叔酰胺的电子密度,从而将 ET 的速率降低多达一个数量级。这些模型研究的结果表明,与单一氨基酸相比,肽中脯氨酸残基对自由基诱导的氧化损伤的敏感性应大大降低。
-
Synthesis of tetrahydrophthalazine and phthalamide (phthalimide) derivatives via palladium-catalysed carbonylation of iodoarenes作者:Diána Marosvölgyi-Haskó、Andrea Petz、Attila Takács、László KollárDOI:10.1016/j.tet.2011.09.095日期:2011.114-Tetrahydrophthalazin-1-one and 1,2,3,4-tetrahydrophthalazin-1,4-dione derivatives were synthesised in high (up to 85%) and low yields using 2-iodobenzyl bromide and 1,2-diiodobenzene as bifunctional substrates, respectively. Iodoarenes, carbon monoxide and various hydrazine derivatives as N-nucleophiles were used in a three-component palladium-catalysed cascade hydrazinocarbonylation. A similar palladium-catalysed1,2,3,4-四氢酞嗪-1-酮和1,2,3,4-四氢酞嗪-1,4-二酮衍生物是使用2-碘代苄基溴和1的高产率(高达85%)和低产率合成的,2-二碘苯分别作为双功能底物。碘芳烃,一氧化碳和各种作为N-亲核试剂的肼衍生物被用于三组分钯催化的级联肼羰基化反应中。类似的钯催化反应,即1,2-二碘苯的氨基羰基化反应,主要导致形成两种主要产物,具体取决于胺N-亲核试剂:使用伯胺可产生N取代的邻苯二甲酰亚胺进行双羰基化,同时仲胺与碘芳烃官能团之一反应,得到相应的2-碘代苯甲酰胺。由于在一个或两个碘代芳烃官能团处插入一氧化碳两次,可以通过改变反应条件来分离出酮羧酰胺-羧酰胺或双酮羧酰胺衍生物。还讨论了闭环反应的一些机理细节和导致副产物的条件。
-
Transamidation of Carboxamides Catalyzed by Fe(III) and Water作者:Liliana Becerra-Figueroa、Andrea Ojeda-Porras、Diego Gamba-SánchezDOI:10.1021/jo500562w日期:2014.5.16The highly efficient transamidation of several primary, secondary, and tertiary amides with aliphatic and aromatic amines (primary and secondary) is described. The reaction is performed in the presence of a 5 mol % concentration of different hydrated salts of Fe(III), and the results show that the presence of water is crucial. The methodology was also applied to urea and phthalimide to demonstrate描述了几种伯,仲和叔酰胺与脂族和芳族胺(伯和仲)的高效氨基转移。反应在浓度为5 mol%的不同水合Fe(III)盐存在下进行,结果表明水的存在至关重要。该方法还应用于尿素和邻苯二甲酰亚胺,以证明其多功能性和广泛的底物范围。其用途的一个实例是分子内应用,用于合成2,3-二氢-5 H-苯并[ b ] -1,4-噻嗪平-4-酮,它是地尔硫卓与结构相关药物的双环核心(Budriesi ,R。; Cosimelli,B。;伊万,P。; Carosati,E。; Ugenti,MP; Spisani,R. CURR医学化学式。2007年,14,279–287)。在实验观察和先前关于由过渡金属(例如铜和铝)催化的氨基转移反应的机理建议的基础上,提出了一种解释水作用的合理机制。与其他现有方法相比,该方法具有显着改进。它可以在空气中和湿或工业级溶剂中进行。
-
Rhodium(iii)-catalyzed oxidative carbonylation of benzamides with carbon monoxide作者:Ya Du、Todd K. Hyster、Tomislav RovisDOI:10.1039/c1cc15843k日期:——An efficient strategy for the oxidative carbonylation of aromatic amidesvia C–H/N–H activation to form phthalimides using an Rh(III) catalyst has been developed. The reaction shows a preference for C–H bonds of electron-rich aromatic amides and tolerates a variety of functional groups.开发了一种通过C-H/N-H活化,利用Rh(III)催化剂高效地将芳香酰胺氧化羰基化合成酞菁的策略。该反应倾向于电子丰富的芳香酰胺的C-H键,并能容忍多种官能团。
-
Development of the Ireland−Claisen Rearrangement of Alkoxy- and Aryloxy-Substituted Allyl Glycinates作者:James P. Tellam、David R. CarberyDOI:10.1021/jo1017124日期:2010.11.19The Ireland−Claisen rearrangement of 3-alkoxy- and 3-aryloxy-substituted allyl glycinates is presented. This [3,3]-sigmatropic rearrangement route offers direct access to syn β-alkoxy and β-aryloxy α-amino acid systems. In particular, N,N-diboc glycine esters rearrange with excellent diastereoselectivities (dr > 25:1). The synthesis of substrates, rearrangement optimization, and a discussion of stereoselection
表征谱图
-
氢谱1HNMR
-
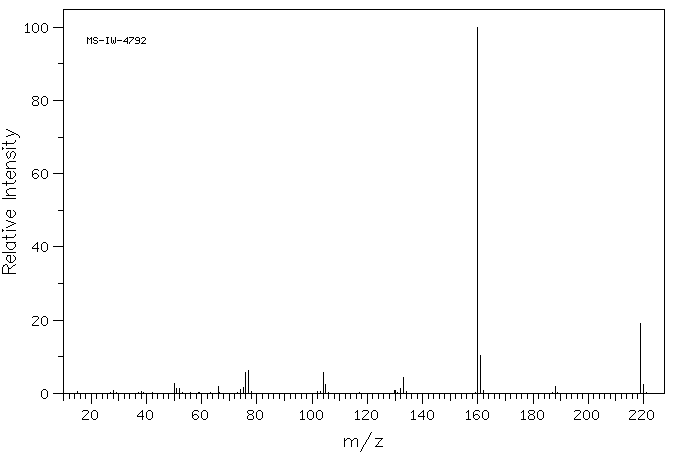
质谱MS
-
碳谱13CNMR
-
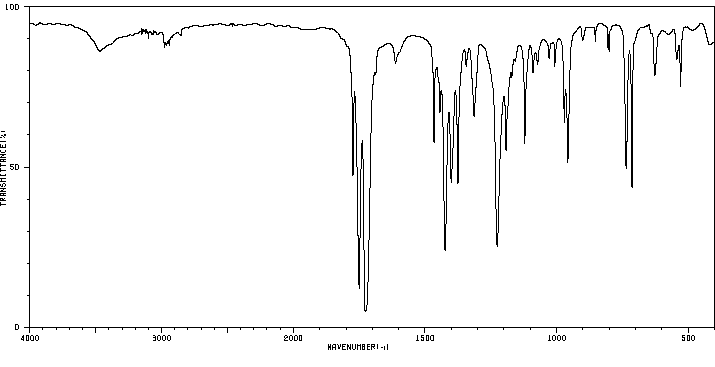
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸