2-(4-氨基苯基)-6-甲基苯并噻唑 | 92-36-4
中文名称
2-(4-氨基苯基)-6-甲基苯并噻唑
中文别名
4-(6-甲基-2-苯并噻唑)苯胺;2-对氨基苯基-6-甲基苯噻唑;脱氢硫代对甲苯胺;2-对氨基苯基-6-甲基苯并噻唑
英文名称
2-(4-Aminophenyl)-6-methylbenzothiazole
英文别名
2-(4-aminophenyl)-6-methyl-1,3-benzothiazole;4-(6-methylbenzo[d]thiazol-2-yl)aniline;4-(6-methyl-1,3-benzothiazol-2-yl)aniline
CAS
92-36-4
化学式
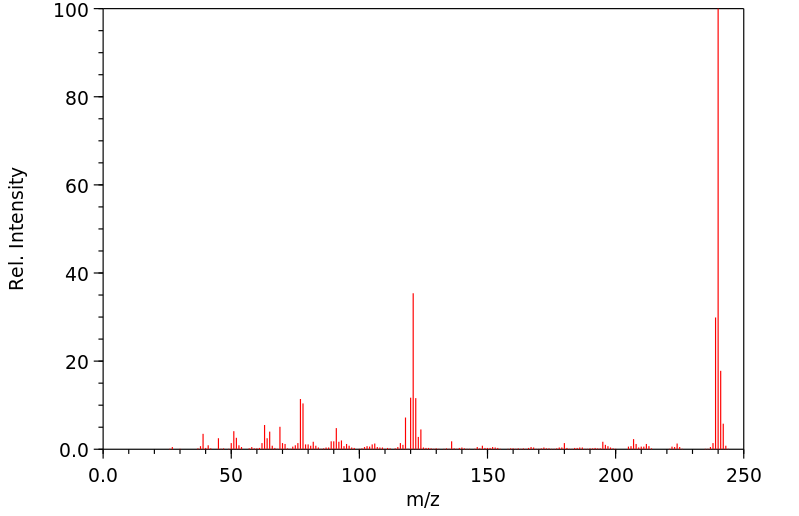
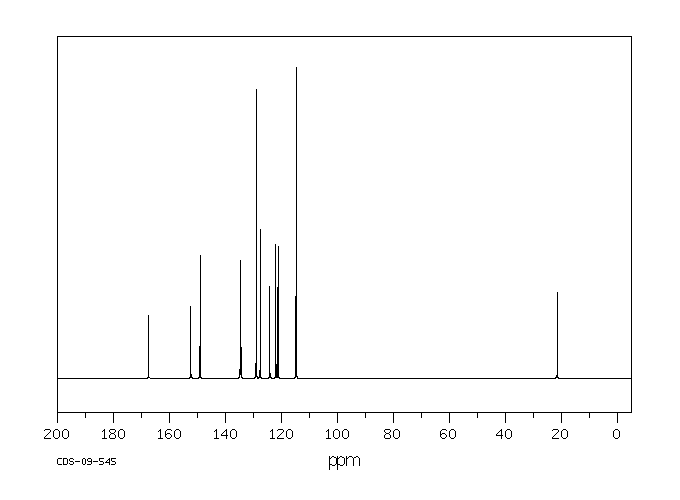
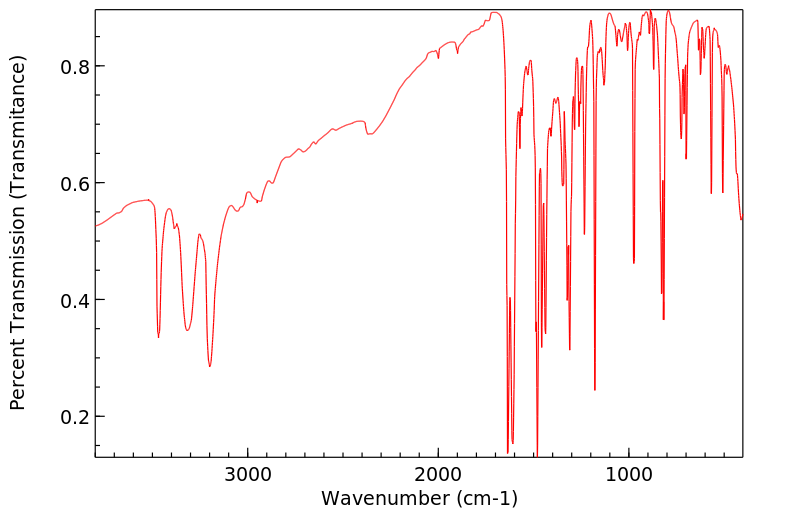
C14H12N2S
mdl
MFCD00005780
分子量
240.329
InChiKey
XRTJYEIMLZALBD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:191-196°C
-
沸点:434°C
-
密度:1.1769 (rough estimate)
-
溶解度:≥ 24mg/mL,溶于 DMSO
-
LogP:3.56 at 25℃
-
物理描述:2-(4-aminophenyl)-6-methylbenzothiazole appears as light tan or light orange powder. Solutions have a violet-blue fluorescence. (NTP, 1992)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温和干燥环境中使用。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-(6-Methyl-2-benzothiazolyl)benzeneamine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(6-Methyl-2-benzothiazolyl)benzeneamine
CAS number: 92-36-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C14H12N2S
Molecular weight: 240.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-(6-Methyl-2-benzothiazolyl)benzeneamine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(6-Methyl-2-benzothiazolyl)benzeneamine
CAS number: 92-36-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C14H12N2S
Molecular weight: 240.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-甲基-2-(4-硝基苯基)苯并噻唑 6-methyl-2-(4-nitrophenyl)benzo[d]thiazole 488722-57-2 C14H10N2O2S 270.312 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(6-methylbenzothiazol-2-yl)phenylhydrazine 774238-55-0 C14H13N3S 255.343 异氰酸4-(6-甲基-2-苯并噻唑基)苯酯 4-(6-methyl-2-benzothiazolyl)phenyl isocyanate 67229-93-0 C15H10N2OS 266.323 2-(4’-(二甲胺基)苯基)-6-甲基苯并噻唑 N,N-dimethyl-4-(6-methylbenzo[d]thiazole-2-yl)aniline 10205-62-6 C16H16N2S 268.382 —— formic acid-[4-(6-methyl-benzothiazol-2-yl)-anilide] 95856-69-2 C15H12N2OS 268.339 —— 1-(4-methoxyphenyl)-3-morpholin-4-ylpropan-1-one,hydrochloride 17205-68-4 C28H20N4S2 476.626 —— 2-(4-Acetylaminophenyl)-6-methylbenzothiazol 10205-61-5 C16H14N2OS 282.366 N,N-二乙基-4-(6-甲基苯并噻唑-2-基)苯胺 N,N-diethyl-4-(6-methylbenzo[d]thiazol-2-yl)aniline 10205-63-7 C18H20N2S 296.436 —— 1-[4-(6-methyl-benzothiazol-2-yl)-phenyl]-3-phenyl-urea 10262-32-5 C21H17N3OS 359.451 —— 1-Methyl-3-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]thiourea —— C16H15N3S2 313.4 2-氯-n-[4-(6-甲基-1,3-苯并噻唑-2-基)苯基]乙酰胺 2-chloro-n-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]acetamide 87992-61-8 C16H13ClN2OS 316.811 —— 2-(4'-Amino-3'-iodophenyl)-6-methylbenzothiazole —— C14H11IN2S 366.22 —— 2-Bromo-4-(6-methyl-2-benzothiazolyl)benzenamine 178804-07-4 C14H11BrN2S 319.22 —— N,N'-bis[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]butanediamide —— C32H26N4O2S2 562.716 —— N,N'-bis[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]hexanediamide —— C34H30N4O2S2 590.77 —— 1-[4-(6-Methyl-1,3-benzothiazol-2-yl)phenyl]-3-prop-2-enylthiourea —— C18H17N3S2 339.5 2'-(对氨基苯基)-6-甲基-2,6'-联苯并噻唑 4-(6-methyl-[2,6']bibenzothiazolyl-2'-yl)-aniline 95-22-7 C21H15N3S2 373.502 4-[6-[6-(6-甲基-1,3-苯并噻唑-2-基)-1,3-苯并噻唑-2-基]-1,3-苯并噻唑-2-基]苯胺 4-(6-methyl-[2,6';2',6'']terbenzothiazol-2''-yl)-aniline 73302-00-8 C28H18N4S3 506.676 —— N-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]benzamide 95856-79-4 C21H16N2OS 344.437 —— N-[2-[2-[2-[2-[3-(ethylamino)-3-oxopropoxy]ethoxy]ethoxy]ethoxy]ethyl]-3-[4-(6-methyl-1,3-benzothiazol-2-yl)anilino]propanamide 1353637-71-4 C30H42N4O6S 586.753 —— N-cyclohexyl-N'-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]urea —— C21H23N3OS 365.5 —— 3-[4-(6-methyl-1,3-benzothiazol-2-yl)anilino]-N-[2-[2-[2-[2-[3-[2-[3-[2-[2-[2-[2-[3-[4-(6-methyl-1,3-benzothiazol-2-yl)anilino]propanoylamino]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]ethylamino]-3-oxopropoxy]ethoxy]ethoxy]ethoxy]ethyl]propanamide 1345331-87-4 C58H78N8O12S2 1143.44 —— 6-methyl-2'-(4-nitro-phenyl)-[2,6']bibenzothiazolyl 855266-37-4 C21H13N3O2S2 403.485 —— 3-hydroxy-N-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]benzamide —— C21H16N2O2S 360.436 —— furan-2-yl-N-[4-(6-methylbenzothiazol-2-yl)phenyl]carboxamide —— C19H14N2O2S 334.398 —— 4-(tert-butyl)-N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)benzenesulfonamide —— C24H24N2O2S2 436.599 —— 2-amino-6-(6-methylbenzothiazol-2-yl)benzothiazole 474966-91-1 C15H11N3S2 297.404 —— tert-butyl 4-(4-(6-methylbenzo[d]thiazol-2-yl)phenylcarbamoyl)piperidine-1-carboxylate 1095340-28-5 C25H29N3O3S 451.59 —— N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)-3-nitrobenzenesulfonamide —— C20H15N3O4S2 425.489 —— 2-imino-3-methyl-6-(6-methylbenzothiazol-2-yl)benzothiazoline 474967-35-6 C16H13N3S2 311.431 —— N-[4-(6-Methylbenzothiazol-2-yl)phenyl]-(2-chloro-5-nitrophenyl)carboxamide 303099-35-6 C21H14ClN3O3S 423.879 —— 4,5-dichloro-N-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]-1,2-thiazole-3-carboxamide 654057-00-8 C18H11Cl2N3OS2 420.343 —— N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)-2-(1-(thiophen-2-ylsulfonyl)piperidin-4-yl)acetamide 1095340-67-2 C24H25N3O2S3 483.679 脱氢硫代对甲苯胺单磺酸 2-(4-aminophenyl)-6-methylbenzo[d]thiazole-7-sulfonic acid 130-17-6 C14H12N2O3S2 320.393 —— N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)-2-(1-(thiophen-2-ylsulfonyl)piperidin-4-yl)acetamide 1095340-66-1 C25H25N3O3S3 511.69 —— N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)-1-(thiophen-2-ylsulfonyl)azetidine-3-carboxamide 1095340-64-9 C22H19N3O3S3 469.609 —— N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)-1-(thiophen-2-ylsulfonyl)pyrrolidine-3-carboxamide 1095340-65-0 C23H21N3O3S3 483.636 —— N-(4-(6-methylbenzo[d]thiazol-2-yl)phenyl)-1-(thiophen-2-ylsulfonyl)piperidine-3-carboxamide 921780-83-8 C24H23N3O3S3 497.663 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:寡价淀粉样蛋白结合剂可减少 SEVI 介导的 HIV-1 感染增强摘要:本文评估了寡价淀粉样蛋白结合分子作为潜在药物的用途,该药物可以减少精液源性病毒感染增强子(SEVI)原纤维对细胞中人类免疫缺陷病毒-1(HIV-1)感染的增强。精液中发现的这些天然存在的淀粉样原纤维被认为是可以促进 HIV-1 病毒颗粒与免疫细胞附着和内化的介质。因此,能够降低 SEVI 在 HIV-1 感染中的作用的分子可能代表了一种降低人类 HIV-1 性传播率的新策略。在这里,我们评估了一组合成的苯并噻唑苯胺(BTA,一种已知的淀粉样蛋白结合分子)寡价衍生物,了解它们与聚集的淀粉样肽协同结合并中和 SEVI 在 HIV-1 感染中的作用的能力。我们证明,这些 BTA 衍生物表现出与聚集淀粉样蛋白的结合随着寡聚物价数增加而增加的总体趋势。重要的是,我们发现与 BTA 单体相比,BTA 寡聚物在减少细胞中 SEVI 介导的 HIV-1 感染方面表现出更好的能力,与之前报道的单体 BTA 衍生物相比,五聚物的功效提高了DOI:10.1021/ja210931b
-
作为产物:描述:6-甲基-2-(4-硝基苯基)苯并噻唑 在 palladium 10% on activated carbon 、 氢气 作用下, 以 二氯甲烷 为溶剂, 反应 4.0h, 以95%的产率得到2-(4-氨基苯基)-6-甲基苯并噻唑参考文献:名称:2-(4-氨基苯基)苯并噻唑衍生物作为光敏剂的合成及生物学评价摘要:使用外源光敏剂的光动力疗法(PDT)目前已被批准用于治疗基底细胞癌(BCC)。2-(4-氨基苯基)苯并噻唑(6)由发色结构组成,可吸收UVA(315-400 nm)中的光。这些结果鼓励我们设计和合成各种2-苯基苯并噻唑(6)。在本文中研究了UVC激活的6在BCC细胞中诱导的光敏效应所涉及的凋亡机制。用6 -UVA处理的细胞显示出一些凋亡特征,包括亚G1群体的增加,膜联蛋白V结合的显着增加以及caspase-3的激活。6-UVA诱导线粒体膜电位(Δ降低ψ公吨),并通过增强的ROS产生和胞外信号调节激酶(ERK)和p38 MAPK表达的促进磷酸ATP。这些结果表明6- UVA在涉及ERK和p38活化的线粒体过程中引起光敏作用,并最终导致BCC细胞凋亡。DOI:10.1016/j.bmc.2010.04.082
文献信息
-
1-Phenyl-<i>N</i>-(benzothiazol-2-yl)methanimine derivatives as Middle East respiratory syndrome coronavirus inhibitors作者:Min-Qi Hu、Heng Li、Ying Lin、Ying Zhang、Jie Tang、Jian-Ping Zuo、Li-Fang Yu、Xian-Kun Tong、Wei Tang、Fan YangDOI:10.1039/d0ra08442e日期:——
A series of novel 1-phenyl-
N -(benzothiazol-2-yl)methanimine derivatives were synthesized and theirin vitro inhibitory potencies were evaluated on MERS-S pseudovirus. -
Discovery and Characterization of Benzimidazole Derivative XY123 as a Potent, Selective, and Orally Available RORγ Inverse Agonist作者:Xishan Wu、Hui Shen、Yan Zhang、Chao Wang、Qiu Li、Cheng Zhang、Xiaoxi Zhuang、Chenchang Li、Yudan Shi、Yanli Xing、Qiuping Xiang、Jinxin Xu、Donghai Wu、Jinsong Liu、Yong XuDOI:10.1021/acs.jmedchem.1c00763日期:2021.6.24(IC50) value of 64 nM and showed excellent selectivity against other nuclear receptors. 27h also potently suppressed cell proliferation, colony formation, and the expression of androgen receptor (AR)-regulated genes in AR-positive prostate cancer cell lines. In addition, 27h demonstrated good metabolic stability and a pharmacokinetic property with reasonable oral bioavailability (32.41%) and moderate half-life受体相关孤儿受体γ(RORγ)已成为治疗癌症和炎症性疾病的一个有吸引力的治疗靶点。在此,我们报告了我们在苯并噻唑和苯并咪唑衍生物作为 RORγ 新型反向激动剂的发现、优化和评估方面所做的努力。代表性化合物27h (命名为XY123)有效抑制RORγ转录活性,半数抑制浓度(IC 50 )值为64 nM,并对其他核受体表现出优异的选择性。 27h还有效抑制 AR 阳性前列腺癌细胞系中的细胞增殖、集落形成和雄激素受体 (AR) 调节基因的表达。此外, 27h表现出良好的代谢稳定性和药代动力学特性,具有合理的口服生物利用度 (32.41%) 和中等的半衰期 ( t 1/2 = 4.98 h)。值得注意的是,口服化合物27h在小鼠22Rv1异种移植肿瘤模型中实现了完全且持久的肿瘤消退。化合物27h可能作为一种新的有价值的先导化合物,用于进一步开发治疗前列腺癌的药物。
-
Synthesis of 4-oxopyrimidinium and 4-oxo-1,4-dihydropyrimidines作者:Irina V. Vedernikova、Achiel Haemers、Yuryi I. RyabukhinDOI:10.1002/jhet.5570360115日期:1999.1The synthesis of N1-substituted 4-pyrimidones is described. These compounds were prepared from their corresponding 4-oxopyrimidinium perchlorates or from a reaction of a primary amine with a N-acyl-β-ketoamide.
-
Synthesis of 2-(4-aminophenyl)benzothiazoles using MF resin supported H+ under solvent free conditions作者:Yingjie Lei、Xinshi Wu、Guochun Zhang、Cuiling AiDOI:10.1134/s1070363215030251日期:2015.3A simple and convenient approach to 2-(4-aminophenyl)benzothiazole derivatives by condensation of o-aminothiophenol with (un)substituted p-aminobenzoic acid under the action of melamine formaldehyde resin (MFR) supported sulfuric acid under microwave irradiation (MW) and solvent-free conditions has been developed. Structures of the corresponding products were elucidated by IR, 1H NMR spectra, and elemental
-
[EN] SUBSTITUTE ISOQUINOLINES USEFUL IN THE TREATMENT OF DISEASES SUCH AS CANCER AND ATHEROSCLEROSIS<br/>[FR] ISOQUINOLINES SUBSTITUEES UTILES POUR LE TRAITEMENT DE MALADIES DU TYPE CANCER ET ATHEROSCLEROSE申请人:GLAXO GROUP LTD公开号:WO2005049576A1公开(公告)日:2005-06-02A compound of Formula (I) wherein: One of R1 and R2 is H and the other represents - NHCONHR4 wherein R4 represents a phenyl or naphthyl group (which may be optionally substituted by one or more substituents independently selected from -C1-6 alkyl, -C1-6 haloalkyl, - CH2CH2CH2-, halogen, C1-6 alkoxy, C1-6 haloalkoxy, OH, NO2), C3-7 cycloalkyl or R4 together with the NH to which it is bonded forms a morpholino group and R3 is H or NHR5 wherein R5 is H, -quinolinyl or -isoquinolinyl, -(CONH)p phenyl (wherein p is 0 or 1 and the phenyl is optionally substituted by one or more substituents independently selected from halogen, -C1-6 alkyl, -C1-6 haloalkyl, -morpholino, -SO2NH2, benzothiazole (substituted by methyl)) or a salt, solvate, or physiologically functional derivative thereof.根据公式(I)的化合物,其中:R1和R2之一是H,另一个代表-NHCONHR4,其中R4代表一个苯基或萘基团(可以任选地被一个或多个独立选自-C1-6烷基,-C1-6卤代烷基,-CH2CH2CH2-,卤素,C1-6烷氧基,C1-6卤代烷氧基,OH,NO2的取代基所取代),C3-7环烷基或R4与它所连接的NH形成一个吗啉基团,并且R3是H或NHR5,其中R5是H,-喹啉基或-异喹啉基,-(CONH)p苯基(其中p是0或1,并且苯基可以任选地被一个或多个独立选自卤素,-C1-6烷基,-C1-6卤代烷基,-吗啉基,-SO2NH2,苯并噻唑(被甲基取代)的取代基所取代)或其盐,溶剂化物,或生理功能衍生物。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z)-1-(3-乙基-5-羟基-2(3H)-苯并噻唑基)-2-丙酮
齐拉西酮砜
齐帕西酮-d8
阳离子蓝NBLH
阳离子荧光黄4GL
锂2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
铜酸盐(4-),[2-[2-[[2-[3-[[4-氯-6-[乙基[4-[[2-(硫代氧代)乙基]磺酰]苯基]氨基]-1,3,5-三嗪-2-基]氨基]-2-(羟基-kO)-5-硫代苯基]二氮烯基-kN2]苯基甲基]二氮烯基-kN1]-4-硫代苯酸根(6-)-kO]-,(1:4)氢,(SP-4-3)-
铜羟基氟化物
钾2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
钠3-(2-{(Z)-[3-(3-磺酸丙基)-1,3-苯并噻唑-2(3H)-亚基]甲基}[1]苯并噻吩并[2,3-d][1,3]噻唑-3-鎓-3-基)-1-丙烷磺酸酯
邻氯苯骈噻唑酮
西贝奈迪
螺[3H-1,3-苯并噻唑-2,1'-环戊烷]
螺[3H-1,3-苯并噻唑-2,1'-环己烷]
葡萄属英A
草酸;N-[1-[4-(2-苯基乙基)哌嗪-1-基]丙-2-基]-2-丙-2-基氧基-1,3-苯并噻唑-6-胺
苯酰胺,N-2-苯并噻唑基-4-(苯基甲氧基)-
苯酚,3-[[2-(三苯代甲基)-2H-四唑-5-基]甲基]-
苯胺,N-(3-苯基-2(3H)-苯并噻唑亚基)-
苯碳杂氧杂脒,N-1,2-苯并异噻唑-3-基-
苯甲酸,4-(6-辛基-2-苯并噻唑基)-
苯甲基2-甲基哌啶-1,2-二羧酸酯
苯并噻唑正离子,2-[3-(1,3-二氢-1,3,3-三甲基-2H-吲哚-2-亚基)-1-丙烯-1-基]-3-乙基-,碘化(1:1)
苯并噻唑正离子,2-[2-[4-(二甲氨基)苯基]乙烯基]-3-乙基-6-甲基-,碘化
苯并噻唑正离子,2-[(2-乙氧基-2-羰基乙基)硫代]-3-甲基-,溴化
苯并噻唑啉
苯并噻唑三氯金(III)
苯并噻唑-d4
苯并噻唑-7-乙酸
苯并噻唑-6-腈
苯并噻唑-5-羧酸
苯并噻唑-5-硼酸频哪醇酯
苯并噻唑-4-醛
苯并噻唑-4-乙酸
苯并噻唑-2-磺酸钠
苯并噻唑-2-磺酸
苯并噻唑-2-磺酰氟
苯并噻唑-2-甲醛
苯并噻唑-2-甲酸
苯并噻唑-2-甲基甲胺
苯并噻唑-2-基磺酰氯
苯并噻唑-2-基甲基-乙基-胺
苯并噻唑-2-基叠氮化物
苯并噻唑-2-基-邻甲苯-胺
苯并噻唑-2-基-己基-胺
苯并噻唑-2-基-(4-氯-苯基)-胺
苯并噻唑-2-基-(4-氟-苯基)-胺
苯并噻唑-2-基-(4-乙氧基-苯基)-胺
苯并噻唑-2-基-(2-甲氧基-苯基)-胺
苯并噻唑-2-基-(2,6-二甲基-苯基)-胺