烟酸己酯 | 23597-82-2
中文名称
烟酸己酯
中文别名
烟酸正己酯;烟酸正已酯
英文名称
hexyl nicotinate
英文别名
Nicotinsaeure-hexylester;n-Hexyl nicotinate;hexyl pyridine-3-carboxylate
CAS
23597-82-2
化学式
C12H17NO2
mdl
MFCD00023587
分子量
207.272
InChiKey
RVYGVBZGSFLJKH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:140°C
-
密度:1,02 g/cm3
-
LogP:3.510
-
溶解度:0.00 M
-
保留指数:1593
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:15
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:39.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933399090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:室温
SDS
Section 1. Chemical Product and Company Identification
Hexyl Nicotinate
Common Name/
Trade Name
Hexyl Nicotinate
Section 4. First Aid Measures
Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least
Eye Contact
15 minutes. Get medical attention if irritation occurs.
Wash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops.
Skin Contact
Serious Skin Contact Not available.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get
medical attention.
Not available.
Serious Inhalation
Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an
Ingestion
unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight
clothing such as a collar, tie, belt or waistband.
Not available.
Serious Ingestion
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
CLOSED CUP: >109°C (228.2°F).
Flash Points
Not available.
Flammable Limits
These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...).
Products of Combustion
Fire Hazards in Presence of Slightly flammable to flammable in presence of open flames and sparks, of heat.
Various Substances
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards in
Risks of explosion of the product in presence of static discharge: Not available.
Presence of Various
Substances
SMALL FIRE: Use DRY chemical powder.
Fire Fighting Media
LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
and Instructions
Not available.
Special Remarks on
Fire Hazards
Not available.
Special Remarks on
Explosion Hazards
Section 6. Accidental Release Measures
Absorb with an inert material and put the spilled material in an appropriate waste disposal.
Small Spill
Absorb with an inert material and put the spilled material in an appropriate waste disposal. Finish cleaning by
Large Spill
spreading water on the contaminated surface and allow to evacuate through the sanitary system.
Hexyl Nicotinate
Section 7. Handling and Storage
Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not
Precautions
breathe gas/fumes/ vapor/spray. Keep away from incompatibles such as oxidizing agents.
Keep container tightly closed. Keep container in a cool, well-ventilated area.
Storage
Section 8. Exposure Controls/Personal Protection
Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
Engineering Controls
respective threshold limit value. Ensure that eyewash stations and safety showers are proximal to the work-station
location.
Personal Protection Safety glasses. Lab coat. Gloves (impervious). Respiratory protection is not necessary for normal handling. Good
room ventilation or use of local exhaust (fume hood) is sufficient. Use a vapor respirator under conditions where
exposure to the substance is apparent (e.g. generation of high concentrations of mist or vapor, inadequate
ventilation, development of respiratory tract irritation), and engineering controls are not feasible. Be sure to use an
approved/certified respirator or equivalent.
Personal Protection in Case Splash goggles. Full suit. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a specialist
of a Large Spill BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Liquid. Not available.
Physical state and O dor
appearance
Not available.
Taste
207.3 g/mole
Molecular Weight
Not available.
Color
Not available.
pH (1% soln/water)
147°C (296.6°F)
Boiling Point
Not available.
Melting Point
Not available.
Critical Temperature
1.02 (Water = 1)
Specific Gravity
Not available.
Vapor Pressure
Not available.
Vapor Density
Not available.
Volatility
Not available.
Odor Threshold
Not available.
Water/Oil Dist. Coeff.
Not available.
Ionicity (in Water)
Not available.
Dispersion Properties
Not available.
Solubility
Section 10. Stability and Reactivity Data
The product is stable.
Stability
Not available.
Instability Temperature
Conditions of Instability Excess heat, incompatible materials
Incompatibility with various Reactive with oxidizing agents.
substances
Hexyl Nicotinate
Not available.
Corrosivity
Not available.
Special Remarks on
Reactivity
Not available.
Special Remarks on
Corrosivity
Will not occur.
Polymerization
Section 11. Toxicological Information
Absorbed through skin. Eye contact.
Routes of Entry
Toxicity to Animals LD50: Not available.
LC50: Not available.
Chronic Effects on Humans Not available.
Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation.
Other Toxic Effects on
Humans
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on Not available.
Chronic Effects on Humans
Special Remarks on other Acute Potential Health Effects:
Skin: May cause skin irritation.
Toxic Effects on Humans
Eyes: May cause eye irritation.
Inhalation: Inhalation of mist or vapor may cause respiratory tract irritation.
Ingestion: Expected to be a low hazard.
Section 12. Ecological Information
Not available.
Ecotoxicity
Not available.
BOD5 and COD
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may
arise.
The product itself and its products of degradation are not toxic.
Toxicity of the Products
of Biodegradation
Not available.
Special Remarks on the
Products of Biodegradation
Section 13. Disposal Considerations
Waste must be disposed of in accordance with federal, state and local environmental control
Waste Disposal
regulations.
Section 14. Transport Information
Not a DOT controlled material (United States).
DO T Cl assi fi cati on
Not applicable.
Identification
Not applicable.
Special Provisions for
Transport
Hexyl Nicotinate
DO T (Pi ctograms)
Section 15. Other Regulatory Information and Pictograms
TSCA 8(b) inventory: Hexyl Nicotinate
Federal and State
Regulations
California prop. 65: This product contains the following ingredients for which the State of California has found to
California
cause cancer which would require a warning under the statute: No products were found.
Proposition 65
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has found to
cause birth defects which would require a warning under the statute: No products were found.
Other Regulations EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No.
245-767-4).
Canada: Listed on Canadian Domestic Substance List (DSL).
China: Not listed on National Inventory.
Japan: Not listed on National Inventory (ENCS).
Korea: Not listed on National Inventory (KECI).
Philippines: Not listed on National Inventory (PICCS).
Australia: Not listed on AICS.
WHMIS (Canada) Not controlled under WHMIS (Canada).
Other Classifications
This product is not classified according Not applicable.
DSCL (EEC)
to the EU regulations.
Health Hazard
HMIS (U.S.A.) 1 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
1 0 Reactivity
Health
Reactivity 0
Specific hazard
Personal Protection
B
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG(Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves (impervious).
Hexyl Nicotinate
Lab coat.
Not applicable.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:Reactivity of perfluoro-(cis-2,3-dialkyloxaziridines) with heteroaromatic nitrogen compounds摘要:在特别温和的条件下,当全氟代二烷基氧杂环丁烷1与在2位带有取代基的吡啶衍生物2反应时,只能生成吡啶N-氧化物3。从在3位和4位被取代的吡啶出发,还会生成并分离得到先前未报道的N-全氟酰基吡啶季铵胺4,它们是固态稳定的化合物。从双吡啶底物以及吡嗪和喹喔啉起始物出发制备的双(吡啶季铵胺)9,也表现出相同的反应活性。这种现象揭示了氧杂环丁烷1既能充当胺化剂也能作为氧化剂的双重功能。DOI:10.1039/p19960002517
-
作为产物:参考文献:名称:Nicotinic Acid. Water-insoluble Esters and Amides摘要:DOI:10.1021/ja01223a028
文献信息
-
Phosphoric Acid Mediated Light‐Induced Minisci C−H Alkylation of <i>N</i> ‐Heteroarenes作者:Songyang Jin、Xinxin Geng、Yujun Li、Ke ZhengDOI:10.1002/ejoc.202001538日期:2021.2.12A visible light‐intduced/phosphate‐mediated light‐induced Minisci‐type reaction under metal‐ and photocatalyst‐free condition was developed, which provided an efficient and environmentally friendly way to access functionalized N‐heteroarenes. The transformation underwent a radical pathway, and the diphenyl phosphate played a key role in the catalytic cycle via hydrogen bonding.
-
一种多配体金属配合物的制备方法及应用申请人:六盘水师范学院公开号:CN108948051A公开(公告)日:2018-12-07
-
Derivative of glucose and of vitamin F, compositions comprising it, uses and preparation process申请人:——公开号:US20040038912A1公开(公告)日:2004-02-26An O-acyl product derived from glucose which may be obtained by partial or total esterification of glucose and of vitamin F, comprising a mixture of esters, for example, monoesters, of glucose and of at least one acid chosen from linoleic acid, oleic acid, palmitic acid and stearic acid, compositions, for example, cosmetic and pharmaceutical compositions, comprising this novel derivative, and their use for improving the condition of head hair and/or other hairs, and, for example, for reducing and/or impeding the loss of head hair and/or other hairs, and/or for inducing and/or stimulating hair growth, as well as a process for preparing O-acyl derivatives mainly in position 6 of glucose, comprising preparing a mixed anhydride by reacting a carboxylic acid with a trimethylacetyl halide, followed by reacting said mixed anhydride formed with glucose.
-
Use of tall oil pitch extract and compositions which contain it申请人:Lipofoods, S.L.公开号:EP2343061A1公开(公告)日:2011-07-13Use of the unsaponificable fraction of tall oil pitch in the preparation of a food, cosmetic and/or pharmaceutical composition for the treatment, care and/or prevention of a disease, disorder and/or condition associated with the 5-α-reductase activity.在食品、化妆品和/或药用组合物的制备中使用松节油焦油的不皂化部分,用于治疗、护理和/或预防与5-α-还原酶活性相关的疾病、紊乱和/或状况。
-
METHOD FOR PRODUCING CARBOXYLIC ACID AMIDE申请人:Tomokawa Junichi公开号:US20130123505A1公开(公告)日:2013-05-16A carboxamide can be produced in a high yield by a method for producing a carboxamide, for example, represented by formula (4): (wherein R 1 and R 3 are as defined below), the method comprising a step of allowing a carboxylic acid ester represented by formula (1): (wherein R 1 represents an optionally substituented C 1 -C 20 hydrocarbon group or an optionally substituented C 3 -C 20 heterocyclic group, and R 2 represents an optionally substituented C 1 -C 20 hydrocarbon group), an amine represented by formula (2): R 3 —NH 2 (2) (wherein R 3 represents a hydrogen atom or an optionally substituented C 1 -C 20 hydrocarbon group), and a formamide compound represented by formula (3): (wherein R 3 is as defined above) to react in the presence of a metal alkoxide.
表征谱图
-
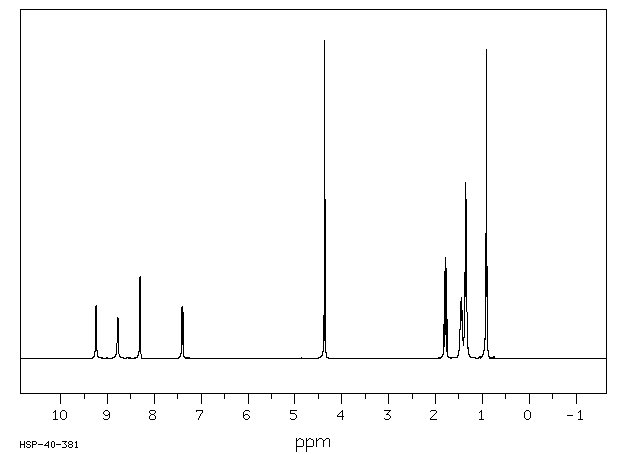
氢谱1HNMR
-
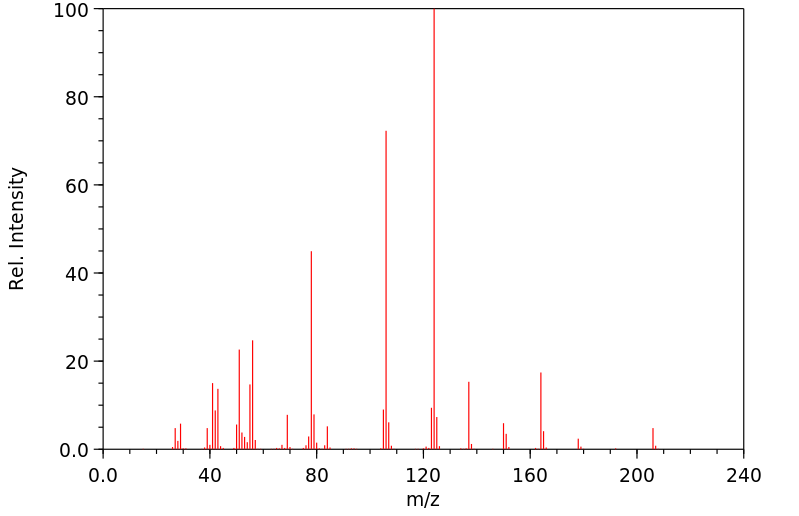
质谱MS
-
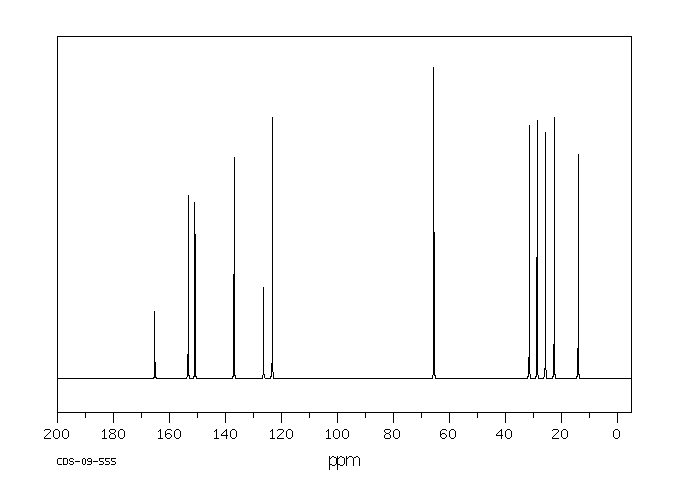
碳谱13CNMR
-
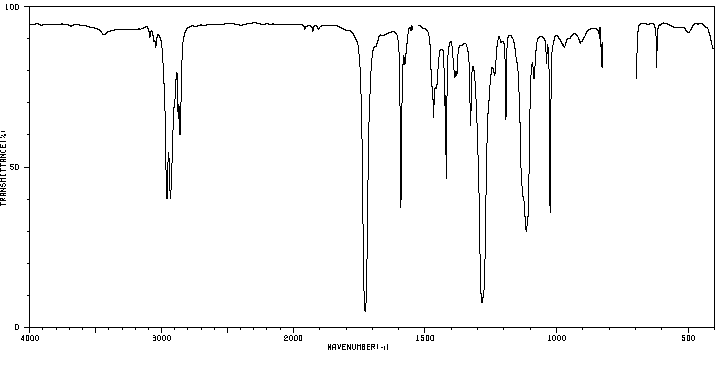
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-