2-胍基苯并咪唑 | 5418-95-1
中文名称
2-胍基苯并咪唑
中文别名
2-胍啶苯并咪唑
英文名称
2-guanidinobenzimidazole
英文别名
1-(1H-benzo[d]imidazol-2-yl)guanidine;N-(1H-benzimidazol-2-yl)guanidine;(2-benzimidazolyl)guanidine;1-(1H-benzimidazol-2-yl)guanidine
CAS
5418-95-1
化学式
C8H9N5
mdl
MFCD00005590
分子量
175.193
InChiKey
JJWCTKUQWXYIIU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:242-244 °C (dec.) (lit.)
-
沸点:296.48°C (rough estimate)
-
密度:1.2954 (rough estimate)
-
溶解度:可溶于 DMSO(最高 50 mg/ml)或水(加热最高 4 mg/ml)
-
稳定性/保质期:
常温常压下稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:93.1
-
氢给体数:3
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2933990090
-
储存条件:密封存储,应置于阴凉干燥的仓库中。
SDS
1.1 产品标识符
: 2-Guanidinobenzimidazole
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C8H9N5
分子式
: 175.19 g/mol
分子量
成分 浓度
1-(Benzimidazol-2-yl)guanidine
-
化学文摘编号(CAS No.) 5418-95-1
EC-编号 226-527-8
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 淡黄
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 242 - 244 °C - 分解
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 2-Guanidinobenzimidazole
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C8H9N5
分子式
: 175.19 g/mol
分子量
成分 浓度
1-(Benzimidazol-2-yl)guanidine
-
化学文摘编号(CAS No.) 5418-95-1
EC-编号 226-527-8
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 淡黄
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 242 - 244 °C - 分解
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-苯并咪唑基间二氮茚基脲 2-benzimidazolylurea 24370-25-0 C8H8N4O 176.178 1H-苯并咪唑-2-基氰胺 2-benzimidazolylcarbamonitrile 55864-37-4 C8H6N4 158.162 —— 1-[(E)-N'-(1H-benzimidazol-2-yl)carbamimidoyl]-3-phenylurea 78650-29-0 C15H14N6O 294.316 —— 2-(1H-benzimidazol-2-ylamino)-1H-pyrimidine-4,6-dione —— C11H9N5O2 243.225
反应信息
-
作为反应物:描述:2-胍基苯并咪唑 以 甲醇 为溶剂, 反应 52.0h, 生成 methyl 2-(2-benzimidazolylamino)-4-oxo-2-imidazolin-5-ylideneacetate参考文献:名称:Furukawa, Mitsuru; Kawanabe, Kimimasa; Yoshimi, Atsuko, Chemical and pharmaceutical bulletin, 1983, vol. 31, # 7, p. 2473 - 2479摘要:DOI:
-
作为产物:描述:参考文献:名称:ABDOU W. M.; MAHRAN M. R.; SIDKY M. M.; WAMHOFF H., CHEMOSPHERE, 15,(1986) N 8, 1063-1071摘要:DOI:
-
作为试剂:描述:参考文献:名称:Reagent And Method For Preparing A Fluorinated and Silylated Derivative摘要:本发明涉及一种制备含有至少一种氟和硅之间的承载碳的键的氟化和硅化衍生物的方法。该方法包括至少一步,在该步骤中,将式(I)的衍生物Rf-CO—O-D,其中D被选择在硅化基团中,置于碱的存在下。本发明的方法用于合成氟化衍生物。公开号:US20070276149A1
文献信息
-
Sulfonylguanidine Derivatives as Potential Antimelanoma Agents作者:Tom Baladi、Nedra Hamouda‐Tekaya、Leticia Christina Pires Gonçalves、Stéphane Rocchi、Cyril Ronco、Rachid BenhidaDOI:10.1002/cmdc.202000218日期:2020.7.3Sulfonylguanidines are interesting bioactive compounds with a broad range of applications in the treatment of different pathologies. 2‐Aminobenzazole‐based structures are well employed in the development of new anticancer drugs. Two series of novel N‐benzazol‐2‐yl‐N′‐sulfonyl guanidine derivatives were synthesized with the sulfonylguanidine in either an extra‐ or intracyclic frame. They were evaluated for their antiproliferative
-
PROCESS FOR STRAIGHTENING KERATIN FIBRES WITH A HEATING MEANS AND DENATURING AGENTS申请人:Philippe Michel公开号:US20100028280A1公开(公告)日:2010-02-04The invention relates to a process for straightening keratin fibres, comprising: (i) a step in which a straightening composition containing at least two denaturing agents is applied to the keratin fibres, (ii) a step in which the temperature of the keratin fibres is raised, using a heating means, to a temperature of between 110 and 250° C.该发明涉及一种直发角蛋白纤维的拉直过程,包括:(i)将至少两种变性剂含有的拉直组合物涂抹到角蛋白纤维上的步骤,(ii)使用加热装置将角蛋白纤维的温度升高至110至250°C的步骤。
-
Anti-allergic five membered heterocyclic ring containing N-(bicyclic申请人:Janssen Pharmaceutica, N.V.公开号:US04634704A1公开(公告)日:1987-01-06Five membered heterocyclic ring containing N-(bicyclic heterocyclyl)-4-piperidinamines having histamine and serotonine antagonistic activity which compounds are useful agents in the treatment of allergic diseases.
-
Pyrroloquinoline Derivatives And Their Use As Protein Kinases Inhibitors申请人:Bienayme Hugues公开号:US20090042876A1公开(公告)日:2009-02-12The present invention relates to inhibitors of protein kinases of formula I: which can be used in the treatment of various diseases, notably cancer, inflammation or disorders of the central nervous system. It also relates to pharmaceutical compositions containing the compounds according to the invention and their use in therapy.本发明涉及式I的蛋白激酶抑制剂,可用于治疗各种疾病,特别是癌症、炎症或中枢神经系统疾病。还涉及含有根据本发明的化合物的药物组合物及其在治疗中的应用。
-
Microwave-Assisted Synthesis of<i>s</i>-Triazino[2,1-<i>b</i>][1,3]benzoxazoles,<i>s</i>-Triazino[2,1-<i>b</i>][1,3]benzothiazoles, and<i>s</i>-Triazino[1,2-<i>a</i>]benzimidazoles作者:Wai-Keung Chui、Anton V. Dolzhenko、Anna V. DolzhenkoDOI:10.1055/s-2006-926290日期:——2-Amino-4-oxo-derivatives of s-triazino[2,1-b][1,3]benzoxazoles, s-triazino[2,1-b][1,3]benzothiazoles, and s-triazino[1,2-a]benzimidazoles were synthesized by carbonylation of 2-benzoxazolylguanidines, 2-benzothiazolylguanidines, and 2-benzimidazolylguanidines with phenyl isocyanate under microwave irradiation (180 °C, 15 minutes). Using phenyl isothiocyanate instead of phenyl isocyanate under the same conditions led to the successful ring closure via thiocarbonylation of 2-benzoxazolylguanidines. However, the formation of 2-imino-4-phenylimino-s-triazino[2,1-b][1,3]benzothiazoles from 2-benzothiazolylguanidines was observed instead under microwave irradiation conditions.
表征谱图
-
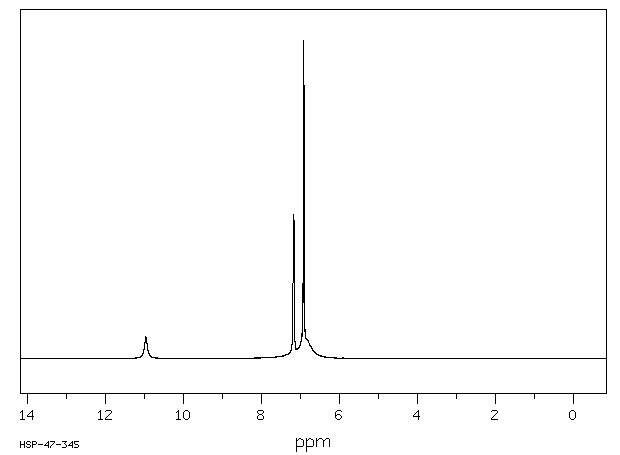
氢谱1HNMR
-
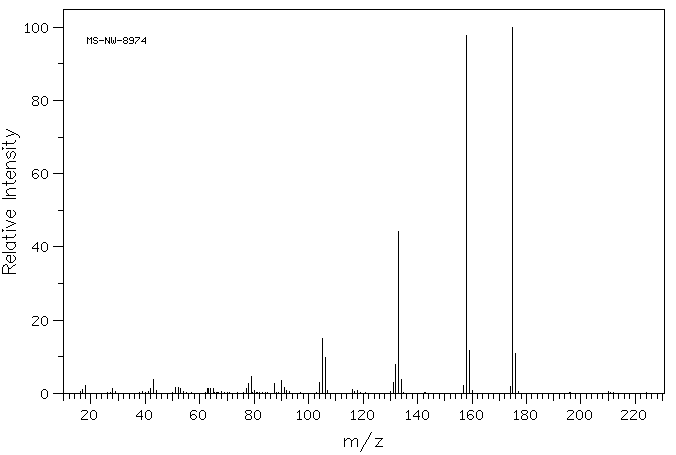
质谱MS
-
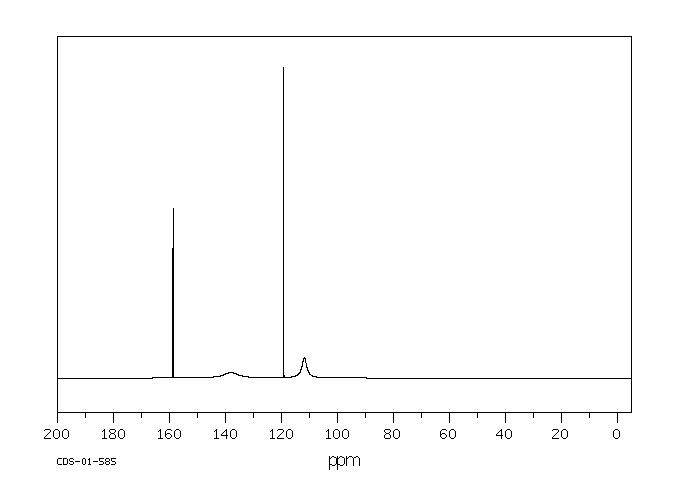
碳谱13CNMR
-
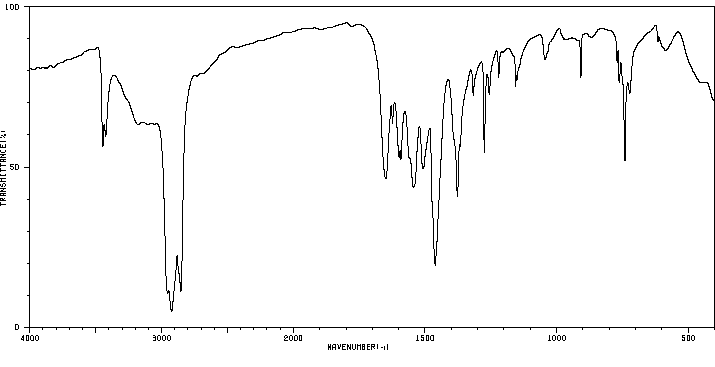
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10