N-Cbz-L-脯氨酸叔丁酯 | 16881-39-3
中文名称
N-Cbz-L-脯氨酸叔丁酯
中文别名
N-苄氧羰基-L-脯氨酸叔丁酯;Z-脯氨酸叔丁基酯;N-CBZ-L-脯氨酸
英文名称
(S)-2-tert-butyl-1-benzylpyrrolidine-1,2-dicarboxylate
英文别名
N-(benzyloxycarbonyl)-L-proline tert-butyl ester;benzyloxy-carbonyl-L-proline tert-butyl ester;(S)-1-Benzyl 2-tert-butyl pyrrolidine-1,2-dicarboxylate;1-O-benzyl 2-O-tert-butyl (2S)-pyrrolidine-1,2-dicarboxylate
CAS
16881-39-3
化学式
C17H23NO4
mdl
——
分子量
305.374
InChiKey
OULMZZGGALAOLR-AWEZNQCLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:45 °C
-
沸点:51 °C / 1mmHg
-
密度:1.151±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:22
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.53
-
拓扑面积:55.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2933990090
-
安全说明:S22,S24/25
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302,H317
-
储存条件:-15°C
SDS
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块 1. 化学品
产品名称: N-Carbobenzoxy-L-proline tert-Butyl Ester
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-苄氧羰基-L-脯氨酸叔丁酯
百分比: >98.0%(LC)(N)
CAS编码: 16881-39-3
俗名: N-Cbz-L-proline tert-Butyl Ester , Z-Pro-OtBu
分子式: C17H23NO4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
45°C
沸点/沸程 51 °C/0.1kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 甲醇
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: N-Carbobenzoxy-L-proline tert-Butyl Ester
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-苄氧羰基-L-脯氨酸叔丁酯
百分比: >98.0%(LC)(N)
CAS编码: 16881-39-3
俗名: N-Cbz-L-proline tert-Butyl Ester , Z-Pro-OtBu
分子式: C17H23NO4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
45°C
沸点/沸程 51 °C/0.1kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 甲醇
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-苄氧羰基-L-脯氨酸叔丁酯 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苄氧羰基-L-脯氨酸 N-Benzyloxycarbonyl-L-proline 1148-11-4 C13H15NO4 249.266 —— (S)-tert-butyl 2-(benzyloxycarbonylamino)-5-bromopentanoate 125050-17-1 C17H24BrNO4 386.286 5-羟基-N-苄氧羰基-L-正缬氨酸叔丁酯 tert-butyl (S)-2-(benzyloxycarbonylamino)-5-hydroxypentanoate 124620-51-5 C17H25NO5 323.389 Z-谷安酸叔丁基酯 (S)-2-(benzyloxycarbonylamino)pentanedioic acid tert-butyl ester 5891-45-2 C17H23NO6 337.373 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-苄氧羰基-L-脯氨酸 N-Benzyloxycarbonyl-L-proline 1148-11-4 C13H15NO4 249.266 (2S)-5-氧代-1,2-吡咯烷二甲酸2-叔丁基1-苄基酯 tert-butyl N-benzyloxycarbonyl-L-pyroglutamate 81470-51-1 C17H21NO5 319.357 1,2-吡咯烷二羧酸,2-(2-羰基乙基)-,2-(1,1-二甲基乙基)1-(苯基甲基)酯 tert-butyl N-benzyloxycarbonyl-2(RS)-(formylmethyl)prolinate 129605-85-2 C19H25NO5 347.411 —— benzyloxycarbonyl-D-valylproline t-butyl ester 21148-60-7 C22H32N2O5 404.506 —— N-<(benzyloxy)acetyl>-L-proline tert-butyl ester 158268-44-1 C18H25NO4 319.401 —— (S)-tert-butyl 1-(2-chloroacetyl)pyrrolidine-2-carboxylate 197453-33-1 C11H18ClNO3 247.722 —— N-pyruvoyl-L-proline-t-butyl ester 52060-81-8 C12H19NO4 241.287 —— leucylproline tert-butyl ester 74509-15-2 C15H28N2O3 284.399 N-苄基-L-脯氨酸叔丁酯 tert-butyl benzyl-L-prolinate 170642-58-7 C16H23NO2 261.364 —— H-Gly-Gly-Ser-Pro-OBut 84552-87-4 C16H28N4O6 372.422 —— tert-butyl (4-methylbenzyl)-L-prolinate 899429-23-3 C17H25NO2 275.391 —— tert-butyl (4-fluorobenzyl)-L-prolinate 899429-25-5 C16H22FNO2 279.355 —— N-<(benzyloxy)acetyl>-L-proline 158268-45-2 C14H17NO4 263.293 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:肽合成中提高的效率和选择性:三乙基硅烷作为碳阳离子清除剂在叔丁酯和叔丁氧羰基保护位点的脱保护中的用途摘要:在二氯甲烷中存在三氟乙酸的情况下,使用三乙基硅烷作为碳正离子清除剂可提高收率,减少反应时间,简化后处理并提高对受保护氨基酸中叔丁酯和叔丁氧羰基位点进行脱保护的选择性在其他对酸敏感的保护基如苄氧羰基,9-芴基甲氧羰基,O-和S-苄基和叔丁硫基存在下的肽。DOI:10.1016/s0040-4039(00)79116-7
-
作为产物:参考文献:名称:制备光学活性保护脯氨酸的简便新方法摘要:用四氢呋喃 (THF) 中的氢化钠处理由受保护的 L-谷氨酸制备的 LN 保护的 2-氨基-5-溴戊酸酯,以高产率得到相应的保护 L-脯氨酸。另一方面,2-氨基丁酸衍生物与氢化钠反应得到1-氨基环丙烷-1-羧酸衍生物。DOI:10.1246/cl.1996.621
文献信息
-
Use of the 4-methoxy-2,6-dimethylbenzenesulfonyl (Mds) group to synthesize dynorphin (1-13) and related peptides.作者:MITSUHIRO WAKIMASU、CHIEKO KITADA、MASAHIKO FUJINODOI:10.1248/cpb.29.2592日期:——The syntheses of a tridecapeptide corresponding to the amino acid sequence of dynorphin [1-13], H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-OH, and related truncated peptides, [4-13], [8-11], and [8-13], using the 4-methoxy-2, 6-dimethylbenzenesulfonyl (Mds) group for the protection of the guanidino function of arginine, are described. To synthesize dynorphin [1-13], three fragments, 1-3, 4-10, and 11-13, were prepared and used as building blocks for the final construction of the amino acid sequence of this peptide. Final deprotection of the fully protected peptide was achieved by treatment with trifluoroacetic acid-thioanisole at 50°C for 2 h and the purification of this synthetic peptide was effected by column chromatography on CM-cellulose. Other truncated peptides, [4-13], [8-11], and [8-13], were synthesized in the same manner as described for dynorphin [1-13]. The applicability of the Mds protecting group for the synthesis of arginine-containing peptides was confirmed.报道了采用4-甲氧基-2,6-二甲基苯磺酰基(Mds)保护精氨酸胍基功能合成与强啡肽[1-13]氨基酸序列相对应的十三肽H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-OH及相关截短肽[4-13]、[8-11]和[8-13]的方法。为合成强啡肽[1-13],制备了三个片段1-3、4-10和11-13,并将其作为构建该肽氨基酸序列的模块。通过在50°C下用三氟乙酸-硫代茴香醚处理2小时来实现完全保护肽的最终脱保护,并且通过CM-纤维素柱色谱纯化这种合成肽。其他截短肽[4-13]、[8-11]和[8-13]以与强啡肽[1-13]相同的方式合成。Mds保护基团适用于含有精氨酸的肽的合成这一应用得到了确认。
-
Ring‐Strain Effects in Base‐Induced Sommelet–Hauser Rearrangement: Application to Successive Stereocontrolled Transformations作者:Eiji Tayama、Kazutoshi Watanabe、Yoshihiro MatanoDOI:10.1002/ejoc.201600611日期:2016.7base-induced Sommelet–Hauser (S–H) rearrangement of azetidine-2-carboxylic acid ester-derived ammonium salts into 2-aryl-substituted derivatives was demonstrated. The ring-strain of four-membered N-heterocycles enables efficient generation of the desired ylide intermediate and enhances the rate of the S–H rearrangement. The asymmetric version of the rearrangement was characterized by excellent levels of
-
Isopropenyl chlorocarbonate (IPCC)1 in amino acid and peptide chemistry: Esterification of N-protected amino acids; Application to the synthesis of the depsipeptide valinomycin作者:Choukri Zeggaf、Joël Poncet、Patrick Jouin、Marie-Noëlle Dufour、Bertrand CastroDOI:10.1016/s0040-4020(01)81083-8日期:1989.1Esterification of N-protected α-amino acids was achieved via isopropenyl chlorocarbonate (IPCC) activation. In situ alcoholysis of the unstable mixed anhydride intermediate was catalyzed by 4-(dimethylamino)pyridine (DMAP). Competing isopropenyl ester formation was negligible when using methylene chloride as the solvent. A variety of esters from primary and secondary alcohols were obtained with good
-
Total synthesis of wewakazole B作者:Bohua Long、Jingzhao Zhang、Xudong Tang、Zhengzhi WuDOI:10.1039/c6ob01783e日期:——Wewakazole B is a novel cyclodecapeptide with highly potent cytotoxic activity isolated from a sample of M. producens collected from the Red Sea. It contains nine common and three modified amino acid residues. The first total synthesis of Wewakazole B was successfully achieved on a gram scale, unambiguously confirming its structure. Notable features include the careful choice of amino acid-protecting
-
Dicarbonates: Convenient 4-Dimethylaminopyridine Catalyzed Esterification Reagents作者:Kazuyoshi Takeda、Akira Akiyama、Hiroko Nakamura、So-ichi Takizawa、Yoshihisa Mizuno、Hiroaki Takayanagi、Yoshihiro HarigayaDOI:10.1055/s-1994-25638日期:——The DMAP (4-dimethylaminopyridine) catalyzed reaction of dialkyl dicarbonates 1a-e and carboxylic acids 2 afforded the corresponding esters 5 in good yield.
表征谱图
-
氢谱1HNMR
-
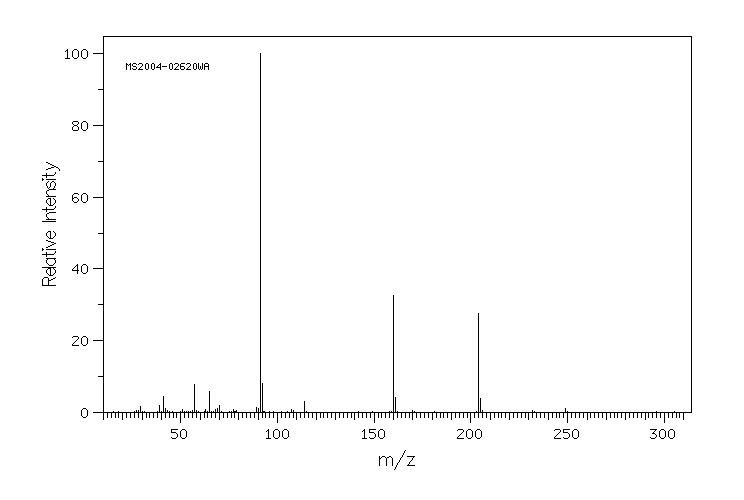
质谱MS
-
碳谱13CNMR
-
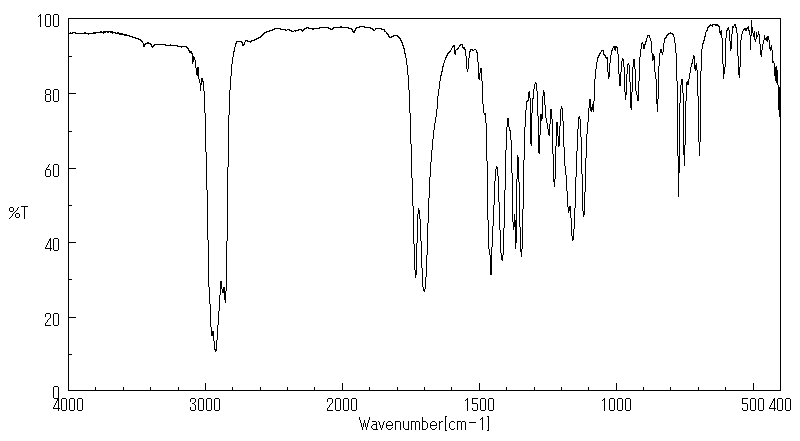
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸