isobutyric acid sodium salt | 996-30-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:320℃
计算性质
-
辛醇/水分配系数(LogP):-3.6
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:40.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915900090
-
危险性防范说明:P261,P264,P270,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P330,P362,P403+P233,P501
-
危险性描述:H302,H312,H332
-
储存条件:2-8°C
SDS
制备方法与用途
适用于生化研究和有机合成。
用途简介暂无具体描述。
反应信息
-
作为反应物:描述:isobutyric acid sodium salt 在 9-borabicyclo[3.3.1]nonane dimer 作用下, 以 四氢呋喃 为溶剂, 反应 1.0h, 以91%的产率得到异丁醛参考文献:名称:Cha, Jin Soon; Oh, Se Yeon; Lee, Kwang Woo, Heterocycles, 1988, vol. 27, # 7, p. 1595 - 1598摘要:DOI:
-
作为产物:描述:参考文献:名称:Biosynthesis of sulfur compounds. Investigations of the biosynthesis of asparagusic acid摘要:DOI:10.1021/ja00294a051
-
作为试剂:描述:苯甲醯亞胺酸 、 在 [(1,2,3,4,5-pentamethylcyclopentadienyl)*Co(CH3CN)3](SbF6)2 、 isobutyric acid sodium salt 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 36.0h, 以53%的产率得到2-[(2,2-dimethyl-3-thiomorpholino-3-thioxopropyl)amino]benzaldehyde参考文献:名称:通过硫酰胺辅助的钴(III)催化的C(sp3)–H活化与蒽进行亲电胺化反应摘要:钴(III)的弱配位硫代酰胺与易于获得的蒽衍生物的惰性C(sp 3)-H键的钴亲电胺化反应在温和的条件下进行,具有良好的官能团耐受性,因此可提供各种氨基醛和氨基酮。此外,我们的协议具有通用性[Cp * Co(MeCN)3 ] [SbF 6 ] 2,具有出色的原子经济性和无氧化剂条件,并且易于后期功能化。DOI:10.1055/s-0039-1690087
文献信息
-
Cost-Benefit Analysis of a Haemophilus Influenzae Type B Meningitis Prevention Programme in The Philippines作者:M. Rhona Limcangco、Carol L. Armour、Eugene G. Salole、Susan J. TaylorDOI:10.2165/00019053-200119040-00006日期:——Background: Haemophilus influenzae type b (Hib) meningitis is associated with high mortality and serious sequelae in children under 5 years of age. Vaccines which can prevent this infection are available. Objective: To evaluate the costs and benefits of a 3-dose immunisation schedule in Manila, Philippines. Perspective: Government and societal perspectives. Design and participants: A cost-benefit analysis based on a birth cohort of 100 000 children. The state of health of the cohort with and without a Hib immunisation programme was modelled over a 5-year period. A survey of medical records of patients with Hib in Manila provided data on the extent and cost of sequelae following infection. Intervention: A 3-dose Hib vaccination programme given at ages 2, 3 and 4 months. Results: The model predicted that vaccinating children against Hib meningitis would prevent 553 cases per year in a birth cohort of 100 000, at a cost of 56 200 Philippine pesos (PHP) [$US1605; 1998 exchange rate] per case (base case assumptions of 90% vaccine efficacy rate, 95 per 100 000 Hib incidence rate, 85% vaccination coverage). Results from the cost-benefit analyses indicated that the saving to the government would be around PHP39 million ($US1.11 million), and the saving to society would be PHP255 million ($US7.28 million). Conclusion: There would be a positive economic benefit for the Philippine government and for the Filipino society if a Hib vaccination programme was introduced in Manila.背景:B 型流感嗜血杆菌(Hib)脑膜炎在 5 岁以下儿童中关联高死亡率和严重后遗症。存在可预防这种感染的疫苗。目标:在菲律宾马尼拉评估三剂接种计划的代价和收益。视角:政府和社会视角。设计和参与者:基于 100,000 名儿童的出生队列进行成本-效益分析。该队列的健康状况在有和没有 Hib 免疫计划的情况下模拟了 5 年的健康状况。一项针对马尼拉 Hib 感染患者的病历调查提供了关于感染后后遗症的严重程度和成本的数据。干预:在 2、3 和 4 个月龄时给予 3 剂 Hib 疫苗接种计划。结果:该模型预测,在 100,000 名出生队列中,预防儿童 Hib 脑膜炎每年将预防 553 例,每例成本为 56,200 菲律宾比索(PHP)[1998 年汇率下为 1,605 美元;基础假设为 90% 疫苗有效率,95/100,000 Hib 发病率,85% 疫苗接种率]。成本-效益分析的结果表明,政府的节约约为 3900 万 PHP(111 万美元),社会的节约约为 25500 万 PHP(728 万美元)。结论:如果在马尼拉引入 Hib 疫苗接种计划,菲律宾政府和菲律宾社会将获得正面的经济收益。
-
SN2 Substitution Reactions at the Amide Nitrogen in the Anomeric Mutagens, N-Acyloxy-N-alkoxyamides作者:Katie L. Cavanagh、Stephen A. Glover、Helen L. Price、Rhiannon R. SchumacherDOI:10.1071/ch09166日期:——amide character and resemble α-haloketones in reactivity. They are susceptible to SN2 reactions at nitrogen, a process that is responsible for their mutagenic behaviour. Kinetic studies have been carried out with the nucleophile N-methylaniline that show that, like SN2 reactions at carbon centres, the rate constant for SN2 displacement of carboxylate is lowered by branching β to the nitrogen centre, or
-
Group Exchange between Ketones and Carboxylic Acids through Directing Group Assisted Rh-Catalyzed Reorganization of Carbon Skeletons作者:Zhi-Quan Lei、Fei Pan、Hu Li、Yang Li、Xi-Sha Zhang、Kang Chen、Xin Wang、Yu-Xue Li、Jian Sun、Zhang-Jie ShiDOI:10.1021/ja512003d日期:2015.4.22The Rh(I)-catalyzed direct reorganization of organic frameworks and group exchanges between carboxylic acids and aryl ketones was developed with the assistance of directing group. Biaryls, alkenylarenes, and alkylarenes were produced in high efficiency from aryl ketones and the corresponding carboxylic acids by releasing the other molecule of carboxylic acids and carbon monoxide. A wide range of functional
-
Catechol derivatives申请人:Hoffmann-La Roche Inc.公开号:US05236952A1公开(公告)日:1993-08-17Catechol derivatives of the formula ##STR1## wherein Ra, Rb and Rc have the significance given herein, the ester and ether derivatives thereof which are hydrolyzable under physiological conditions and the pharmaceutically acceptable salts thereof are described and possess valuable pharmacological properties. In particular, they inhibit the enzyme catechol-O-methyltransferase (COMT), a soluble, magnesium-dependent enzyme which catalyses the transference of the methyl group of S-adensoylmethionine to a catechol substrate, whereby the corresponding methyl ethers are formed. Suitable substrates which can be O-methylated by COMT and which can thus be deactivated are, for example, extraneuornal catecholamines and exogeneously-administered therapeutically active substances having a catechol structure. Formula Ia above embraces not only compounds which form part of the invention, but also known compounds; the compounds which form part of the invention can be prepared according to known methods.
-
Thiocyano ester申请人:ROHM &公开号:US02220521A1公开(公告)日:1940-11-05
537,815. Esters ; insecticides. ROHM &; HAAS CO. Oct. 2, 1939, No. 27025. Convention date, Nov. 8, 1938. [Class 2 (iii)] [Also in Group VI] Esters of unsubstituted glycols or polyalkylene glycols, in which one hydroxyl group is esterified with a monocarboxylic acid containing at least four carbon atoms and the other is replaced by a thiocyano radicle, are made by heating the monocarboxylic acid ester of a halohydrin with a thiocyanate. The monocarboxylic ester of the halohydrin may be prepared by esterifying the glycol (1 mol.) with the acid (1 mol.) and treating the product with phosphorus trichloride, by reacting a halide of the acid with an alkylene oxide or halohydrin, by reacting the sodium salt of the acid with an alkylene dihalide, or by esterifying the acid with a halohydrin. The replacement of the halogen atom by a thiocyano group may be effected by heating with excess of an anhydrous inorganic thiocyanate in the presence or absence of an anhydrous solvent, e.g. methyl isobutyl ketone, copper or sodium .iodide being used as a catalyst if desired. The monocarboxylic acids may be aliphatic, aromatic, arylaliphatic, cycloaliphatic or heterocyclic, the following being specified : butyric, isobutyric, crotonic, α-ethylbutyric, capric, caprylic, lauric, α-ethyl-hexoic, myristic, naphthenic, benzoic, benzyloxybenzoic, salicylic, clupanodonic, chlorobenzoic. phenylacetic, abietic, campholic, naphthylacetic, tetrahydronaphthylacetic, fluorobenzoic, oleic, linoleic, eloidic, ricinoleic, octyloxyacetic, caprylphenoxyacetic, cyclohexyloxyacetic, m-nitrobenzoic, benzoylbenzoic, acetoacetic, undecylenic, stearic, eleostearic, palmitic, and commercial fatty acid mixtures, e.g. coco-nut oil fatty acids, mixtures obtained by oxidation of petroleum, and tall oils. As glycols, ethylene, propylene and butylene glycols, 1 : 10-decanediol, and diethylene ; triethylene and dibutylene glycols are specified. In examples products are obtained from (1) ethylene chlorhydrin, sodium thiocyanate and (a) furoic acid, (b) benzoic acid, (c) lauric acid, (d) technical coco-nut oil acid, (e) a commercial mixture of fatty acids obtained by oxidation of petroleum, and (f) naphthenic acid, (2) sodium isobutyrate is heated with #: #<;SP>;1<;/SP>;-dichlorodiethyl ether, and the product heated with sodium thiocyanate, and (3) the sodium salt of coco-nut oil acid is heated with #: #<;SP>;1<;/SP>;-dichlorodiethyl ether, and the product heated with sodium thiocyanate. The products are useful as insecticides ; they may be dissolved in kerosene to produce insect sprays, or mixed with talc to produce insecti- .cidal dusting powders. They may also be dissolved in a light oil and emulsified in water to produce agricultural sprays. They may be used in admixture with other toxic materials, such as other organic thiocyanates, rotenone, derris extract, pyrethrum, nitro-substituted phenylbenzyl ethers, or nitro-substituted diphenyl ethers. Specification 361,900 is referred to.
537,815. 酯类;杀虫剂。ROHM & HAAS CO. 1939年10月2日,编号27025。公约日期,1938年11月8日。[2类(iii)] [也在第六组中] 未取代的乙二醇或聚烷基乙二醇的酯类,其中一个羟基与至少含有四个碳原子的一元羧酸酯化,另一个被硫氰基取代,通过将一元羧酸酯与硫氰酸盐加热制备。一元羧酸酯可以通过将乙二醇(1摩尔)与酸(1摩尔)酯化并用三氯化磷处理产物,通过将酸的卤化物与烷氧化物或卤水合物反应,通过将酸的钠盐与烷二卤化物反应,或通过将酸与卤水合物酯化来制备。将卤素原子替换为硫氰基可以通过在无水无机硫氰酸盐的过量存在下加热,在无水溶剂的存在或缺席下进行,例如甲基异丁基酮,如果需要,可使用铜或钠碘化物作为催化剂。一元羧酸可以是脂肪的、芳香的、芳基脂肪的、环脂肪的或杂环的,具体如下:丁酸、异丁酸、巴豆酸、α-乙基丁酸、癸酸、辛酸、月桂酸、α-乙基己酸、肉豆蔻酸、环烷酸、苯甲酸、苄氧基苯甲酸、水杨酸、鱼腥草酸、氯苯甲酸、苯乙酸、松香酸、萜酸、萘乙酸、四氢萘乙酸、氟苯甲酸、油酸、亚油酸、油酸、蓖麻油酸、辛氧基乙酸、辛基苯氧乙酸、环己氧基乙酸、m-硝基苯甲酸、苯甲酰苯甲酸、乙酰乙酸、十一烯酸、硬脂酸、亚油酸、棕榈酸和商业脂肪酸混合物,例如椰子油脂肪酸、由石油氧化得到的混合物和松香油。作为乙二醇,指定了乙烯、丙烯和丁烯乙二醇、1:10-癸二醇和二乙二醇;三乙二醇和二丁烯乙二醇。在示例中,从(1)乙烯氯水合物、硫氰酸钠和(a)呋酸、(b)苯甲酸、(c)月桂酸、(d)技术椰子油酸、(e)由石油氧化得到的商业脂肪酸混合物和(f)环烷酸中获得产品,(2)异丁酸钠与#:#<;SP>;1<;/SP>;-二氯二乙醚加热,产物与硫氰酸钠加热,以及(3)椰子油酸钠盐与#:#<;SP>;1<;/SP>;-二氯二乙醚加热,产物与硫氰酸钠加热。产品可用作杀虫剂;它们可以溶解在煤油中制成杀虫喷雾,或与滑石混合以制成杀虫粉末。它们也可以溶解在轻质油中,并乳化在水中制成农业喷雾。它们可以与其他有毒材料混合使用,例如其他有机硫氰酸盐、罗汤宁、德里斯提取物、除虫菊、硝基取代的苯基苯基醚或硝基取代的二苯基醚。参考规范361,900。
表征谱图
-
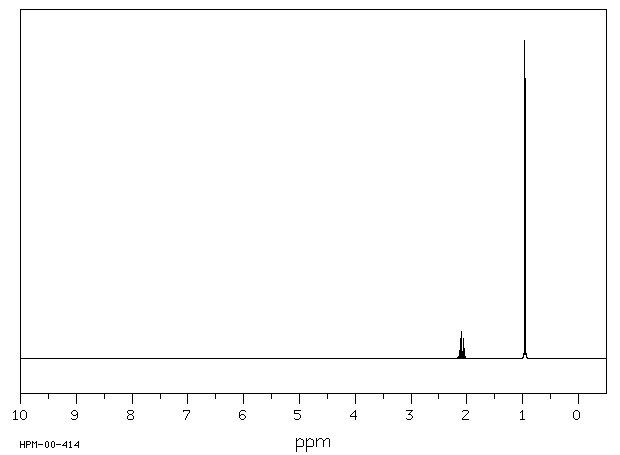
氢谱1HNMR
-
质谱MS
-
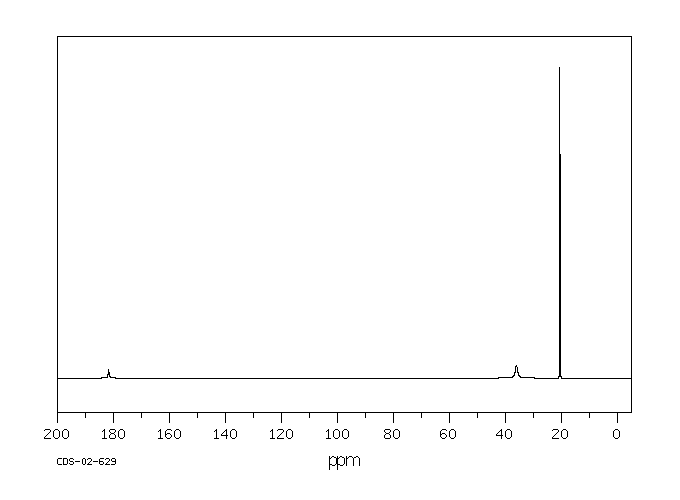
碳谱13CNMR
-
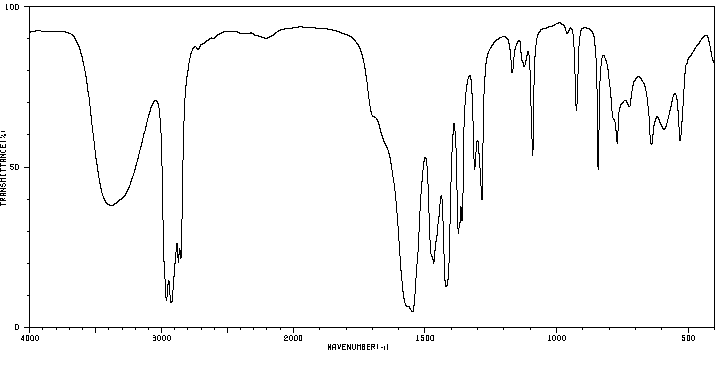
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息