二(甲烷磺酰基)胺 | 5347-82-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:152-153 °C
-
沸点:307.3±25.0 °C(Predicted)
-
密度:1.540±0.06 g/cm3(Predicted)
-
溶解度:1.10 M
计算性质
-
辛醇/水分配系数(LogP):-1.1
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:97.1
-
氢给体数:1
-
氢受体数:5
安全信息
-
海关编码:2935009090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二(甲基磺酰基)羟胺 N,N-bis(methylsulfonyl)hydroxylamine 96088-55-0 C2H7NO5S2 189.213 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-methyl-dimesylamine 3989-37-5 C3H9NO4S2 187.241
反应信息
-
作为反应物:参考文献:名称:Methyl NFSI: atom-economical alternative to NFSI shows higher fluorination reactivity under Lewis acid-catalysis and non-catalysis摘要:Me-NFSI对于活性甲基的氟化在Lewis酸催化和非催化条件下比NFSI更有效。DOI:10.1039/c5gc02612a
-
作为产物:描述:N-(benzyl)dimethyldisulfonimide 在 palladium on activated charcoal 氢气 作用下, 以 四氢呋喃 为溶剂, 反应 1.0h, 以100%的产率得到二(甲烷磺酰基)胺参考文献:名称:聚二磺酰亚胺的合成及反应性摘要:首次合成烷基二磺酰亚胺低聚物。在合成这些低聚物的过程中,发现了以前未报道的 N-取代二磺酰亚胺官能团的反应性。在碱性条件下,低聚物通过“转二磺酰亚胺化”反应发生意外延长,由此通过与磺酰胺阴离子反应从现有二磺酰亚胺合成新的二磺酰亚胺。该过程似乎是通过形成亚砜中间体来进行的。对 E1cB(Rev) 机制的支持包括同位素加扰、取代基效应和亚砜捕获。DOI:10.1021/ja002465v
-
作为试剂:参考文献:名称:Blaschette, Armand; Wieland, Elke; Hamann, Thomas, Zeitschrift fur Naturforschung, B: Chemical Sciences, 1992, vol. 47, # 12, p. 1693 - 1700摘要:DOI:
文献信息
-
Iodine(III)-Promoted Intermolecular Diamination of Alkenes作者:José A. Souto、Yolanda González、Alvaro Iglesias、Debora Zian、Anton Lishchynskyi、Kilian MuñizDOI:10.1002/asia.201101025日期:2012.5A rapid and productive vicinal diamination of alkenes takes place in the presence of a hypervalent iodine(III) reagent and bissulfonimides as nitrogen sources. A total of more than 60 examples are presented. The reaction is characterized by its robustness and its wide substrate scope: it proceeds selectively with both terminal and internal alkenes and tolerates a range of functional groups.
-
Polysulfonylamine, CXXVII [1] Wasserstoffbrücken in kristallinen Onium-dimesylamiden: Ein robustes Achtring-Synthon als Bestandteil dreidimensionaler Wasserstoffbrückenmuster / Polysulfonylamines, CXXVII [1] Hydrogen Bonding in Crystalline Onium Dimesylamides: A Robust Eight-Membered Ring Synthon as a Component in Three-Dimensional Hydrogen Bonding Patterns作者:Karna Wijaya、Oliver Moers、Dagmar Henschel、Armand Blaschette、Peter G. JonesDOI:10.1515/znb-2000-0813日期:2000.8.1
Abstract In order to study crystal packings and hydrogen bonding frameworks, low-temperature X-ray structures were determined for three onium salts of general formula B H+(MeSO2)2N−, where BH+ is acetamidinium (1, monoclinic, space group P21/c, Z = 4), guanidinium (2, monoclinic, P21/c, Z = 4), or 4,6-diamino-2-thioxo-2,3-dihydropyrimidinium (3, monoclinic P21/n, Z = 4). In every case the ions create three-dimensional N − H ∙∙∙ N/O networks, in which all N − H donors of the cations and four (in 1) or five (2, 3) acceptors of the anion are involved; non-conventional secondary bonds, e.g. C − H ∙∙∙ O/N, do not play an important role in the packings. For the isotypic structures 1 and 2, congruencies and dissimilarities of the hydrogen bonding patterns are discussed in detail. Despite the structural and chemical differences between the cations and the way in which they are attached to the anion, each of the three structures displays a robust eight-membered ring synthon [N2 = R2 2(8), antidromic] constructed via two-centre hydrogen bonds from a syn, syn-sequence H − N − C (sp2) −N −H of the respective cation and a V-shaped O − S (sp3) − N fragment of the anion. Although the networks in 2 and 3 contain several N − H (∙∙∙ O)2 three-centre interactions, the two-point connectivity of the synthon is distinctly preserved. The results indicate that the (MeSO2)2N− ion may be utilized in crystal engineering as a dependable building block.
为了研究晶体的堆积和氢键框架,对三种通式为BH+(MeSO2)2N−的醇盐进行了低温X射线结构测定,其中BH+分别为乙酰胺盐(1,单斜,空间群P21/c,Z = 4)、胍盐(2,单斜,P21/c,Z = 4)或4,6-二氨基-2-硫代-2,3-二氢嘧啶盐(3,单斜P21/n,Z = 4)。在每种情况下,离子形成三维N − H ∙∙∙ N/O网络,其中阳离子的所有N − H供体和阴离子的四个(在1中)或五个(2,3)受体参与;非常规的二次键,例如C − H ∙∙∙ O/N,在堆积中并不起重要作用。对于同型结构1和2,详细讨论了氢键模式的一致性和差异性。尽管阳离子之间存在结构和化学上的差异以及它们与阴离子连接的方式不同,但三种结构都显示出由阳离子的同构同向序列H − N − C(sp2)−N −H和阴离子的V形O − S(sp3)−N片段通过双中心氢键构建的稳定的八元环合子[N2 = R22(8),反向]。尽管2和3中的网络包含几个N − H (∙∙∙ O)2三中心相互作用,但合子的双点连接性得到明显保留。结果表明(MeSO2)2N−离子可以作为可靠的构建模块用于晶体工程。 -
An Approach to the Regioselective Diamination of Conjugated Di- and Trienes作者:Anton Lishchynskyi、Kilian MuñizDOI:10.1002/chem.201103435日期:2012.2.20It's do or diaminate: The selective diamination of 1,3‐butadienes in the presence of hypervalent iodine reagents has been developed. This oxidation process proceeds with complete selectivity in favor of diamination. Depending on the substrate, it proceeds either with 1,2‐ or 1,4‐regioselectivity (see scheme).
-
Oxidative Diamination Promoted by Dinuclear Iodine(III) Reagents作者:Caren Röben、José A. Souto、Eduardo C. Escudero-Adán、Kilian MuñizDOI:10.1021/ol3034884日期:2013.3.1New dinuclear iodine(III) reagents for the intermolecular diamination of alkenes are reported. These are accessible through protolytic aminolysis events, which generate defined imido-iodine(III) groups.
-
Thermochemical analysis of solvate complexes of silver dimesylaminide作者:H.K. Cammenga、M. Epple、A. Blaschette、M. NävekeDOI:10.1016/0040-6031(89)85346-8日期:1989.9and calorimetric methods (thermogravimetry, differential scanning calorimetry, thermooptical analysis, solution calorimetry). The hydrate AgN (SO 2 CH 3 ) 2 · 1 4 H 2 O loses its crystal water above 120°C. The dehydratation is overlapped by a phase transition which occurs at 174°C. The acetonitrile complex AgN(SO 2 CH 3 ) 2 ·2 CH 3 CN decomposes between −15 and 95°C. The existence of the hydrate complex
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
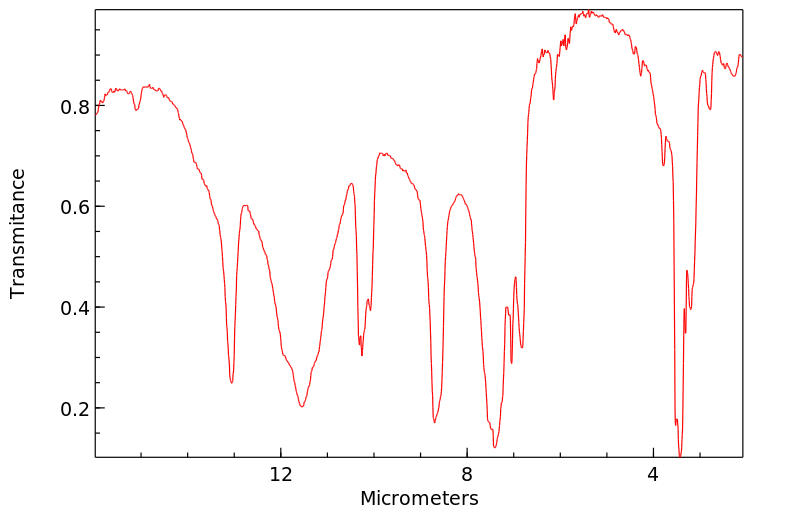
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息