2-甲基-2,4-戊二醇 | 107-41-5
中文名称
2-甲基-2,4-戊二醇
中文别名
α,α,α'-三甲基三亚甲基乙二醇;2-甲基戊烷-2,4-二醇;异己二醇;己烯二醇;环己二醇;己二醇;MPD;(±)-2-甲基-2,4-戊二醇;HG;己烯乙二醇;亚己基二醇
英文名称
2-methyl-2,4-pentanediol
英文别名
2-methylpentane-2,4-diol;hexylene glycol
CAS
107-41-5
化学式
C6H14O2
mdl
——
分子量
118.176
InChiKey
SVTBMSDMJJWYQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-40 °C (lit.)
-
沸点:197 °C (lit.)
-
密度:0.925 g/mL at 25 °C (lit.)
-
蒸气密度:4.1 (vs air)
-
闪点:201 °F
-
溶解度:H2O:1 Mat 20 °C,透明,无色
-
最大波长(λmax):λ: 260 nm Amax: 0.01λ: 280 nm Amax: 0.01
-
介电常数:24.399999999999999
-
暴露限值:ACGIH: TWA 25 ppm; STEL 50 ppm(10 mg/m3)NIOSH: Ceiling 25 ppm(125 mg/m3)
-
LogP:0 at 20℃
-
物理描述:Hexylene glycol is an oily colorless liquid with a mild sweet odor. Floats and mixes slowly with water. (USCG, 1999)
-
颜色/状态:Liquid
-
气味:Mild sweetish
-
蒸汽密度:Relative vapor density (air = 1): 4.1
-
蒸汽压力:Vapor pressure = 0.013 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 4.06X10-7 atm-cu m/mol at 25 °C (est)
-
稳定性/保质期:
-
自燃温度:306 °C
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
粘度:34 cP at 20 °C
-
燃烧热:Standard net heat of combustion = -3.4356x10+9 J/kmol
-
汽化热:13.7 kcal/mol at the boiling point
-
表面张力:33.1 dyne/cm at 20 °C
-
气味阈值:Odor Threshold Low: 3.93 [mmHg]; Odor threshold of 3.93 reported
-
折光率:Index of refraction: 1.4276 at 20 °C/D
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
ADMET
代谢
...Five human subjects ... /had/ both free & conjugated hexylene glycol in the urine after single or repeated oral doses. ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
己二醇喂养给兔子后,尿液中含有7种代谢物,包括己二醇的葡萄糖苷酸(占剂量的46%)、未改变的己二醇(2.5%)、双丙酮醇(1.4%)以及一种未识别的葡萄糖苷酸,可能是双丙酮醇的共轭物。通过与大鼠肝片的孵化,己二醇转化为了双丙酮醇。
(14)C-hexylene glycol fed to rabbits... urine contained 7 metabolites incl glucuronide of hexylene glycol (46% of dose), unchanged hexylene glycol (2.5%), diacetone alcohol (1.4%) & an unidentified glucuronide which could be conjugate of diacetone alcohol. ...Converted into diacetone alc by incubation with rat liver slices.
来源:Hazardous Substances Data Bank (HSDB)
代谢
口服己二醇给大鼠和兔子,导致血浆和尿液中的己糖酸含量显著增加。
... Oral administration of hexylene glycol to rats & rabbits resulted in a substantial increase in the amt of hexuronates in the plasma & in the urine.
来源:Hazardous Substances Data Bank (HSDB)
代谢
It was also shown that approximately 40% of the hexylene glycol was accounted for in the urine, but only 4% of the amount excreted was free glycol; the other 36% was conjugated with glycuronic acid.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:己二醇是一种无色液体,用作化学中间体,石油精炼中的选择性溶剂,液压油的组成部分,油墨的溶剂,水泥添加剂,以及化妆品中。人类暴露和毒性:五名人类受试者每天口服己二醇37克,持续24天(估计每日剂量为14-28毫克/千克体重),没有报告可以归因于摄入己二醇的主观症状;尿液参数没有发生变化。在另一项研究中,大多数受试者在空气中暴露于50 ppm的己二醇15分钟后能够检测到气味,少数人注意到眼睛刺激。在100 ppm的浓度下,气味明显,有些人注意到鼻腔刺激和呼吸道不适。在1000 ppm(4840 mg/立方米)的浓度下,眼睛、鼻子和喉咙有刺激感,以及呼吸道不适。此外,还有报道称,在一例严重接触性荨麻疹并发全身性反应,类似于过敏反应,在使用含有己二醇作为辅料的外用皮质类固醇后发生。在48小时内使用30%或50%水溶液进行的覆盖贴片测试中,23名湿疹患者中出现了肿胀和发红。动物毒性研究:动物急性中毒的临床症状主要是中枢神经系统(CNS)抑制,包括活动减少、肌肉失调和弛缓、眼睑闭合、毛发直立、麻醉和昏迷。将己二醇以20 mg/天的方式口服给予小鼠,持续81天,只在少数动物的肾脏中发现了轻微的影响。在4个月内以平均98和150 mg/天的速率喂食己二醇的大鼠在生长方面没有出现不良反应,肝脏和睾丸也没有组织病理学变化,但肾脏有轻微变化。还表明,大约40%的己二醇在尿液中得到证实,但只有4%的排泄量是自由的甘油;其他36%与葡萄糖醛酸结合。在另一项研究中,己二醇通过口服灌胃的方式给予Sprague Dawley大鼠,剂量水平为50、150和450 mg/kg/天,持续90天。这项研究包括功能性观察测试(FOB),没有发现神经毒性的证据。在450 mg/kg/天的剂量下,在两性中观察到肝细胞增生伴随肝脏重量增加,在150 mg/kg/天的剂量下,仅在雄性中观察到。在没有退行性或坏死的改变的情况下,这被认为是增加代谢需求的适应性反应。在150和450 mg/kg/天的剂量下,仅在雄性大鼠中观察到肾组织病理学(肾小管上皮细胞中酸性颗粒的发病率和高严重性)和肾脏重量增加,这提示了雄性大鼠特有的α-2-微球蛋白肾病,随后得到了证实。对其他器官,包括生殖器官没有不良反应。当给予雄性大鼠平均口服剂量为148至190 mg/天的己二醇130天后,与对照 group相比,生育力没有变化。在大鼠发育研究中,发现在1000 mg/kg/天的剂量下,幼崽死亡率增加,体重增长减少。己二醇对鼠伤寒沙门氏菌TA 1535、TA 1537、TA 1538、TA 98、TA 100的Ames试验结果为阴性,无论是否激活。生态毒性研究:通过抑制三胸苷酸在暴露于有毒化学物质后的结合,评估了对海胆胚胎(Arbacia punctulaTA)的水生毒性。在包括己二醇在内的化合物的初步试验中,结果准确地预测了在48小时暴露后存活率和形态学延迟的减少。
IDENTIFICATION AND USE: Hexylene glycol is a colorless liquid used as a chemical intermediate, a selective solvent in petroleum refining, a component of hydraulic fluids, a solvent for inks, a cement additive, and in cosmetics. HUMAN EXPOSURE AND TOXICITY: Five human subjects given oral doses of 37 g of hexylene glycol daily for 24 days (estimated daily dosage 14-28 mg/kg body weight) reported no subjective symptoms that could be attributed to the intake of hexylene glycol; no alterations in urine parameters were detected. In another study, most subjects exposed for 15 min to 50 ppm of hexylene glycol in the air were able to detect the odor and a few noted eye irritation. At a concentration of 100 ppm, the odor was plain and some noted nasal irritation and respiratory discomfort. At 1000 ppm (4840 mg/cu m), there was irritation of the eyes, nose, and throat, and respiratory discomfort. Additionally, a case of severe contact urticaria with systemic involvement resembling an anaphylactic reaction, following the application of a topical corticosteroid formulated with hexylene glycol as an excipient has been reported. Swelling and redness were seen in 23 out of 823 eczema patients who were treated with a 30% or 50% aqueous solution in a 48-hr covered patch test. ANIMAL TOXICITY STUDIES: The clinical signs observed in animals acutely intoxicated with hexylene glycol are predominately of central nervous system (CNS) depression and include decreased activity, muscle incoordination and flaccidity, palpebral closure, piloerection, narcosis and anesthesia. Mice were fed hexylene glycol orally 20 mg/day in 2 mL of whole milk for up to 81 days and only minor effects were found in the kidneys of a few animals. None of the rats that were fed hexylene glycol in milk for 4 months at the average rate of 98 and 150 mg/day showed adverse effects in growth nor were there histopathologic changes in the liver and testes, but there were minor changes in the kidneys. It was also shown that approximately 40% of the hexylene glycol was accounted for in the urine, but only 4% of the amount excreted was free glycol; the other 36% was conjugated with glycuronic acid. In another study, hexylene glycol was administered by oral gavage for 90 days to Sprague Dawley rats at dose levels of 50, 150 and 450 mg/kg/day. This study included a functional observational battery (FOB), which gave no evidence of neurotoxic effects. Hepatocellular hypertrophy coupled with increased liver weight was observed at 450 mg/kg/day in both sexes and in males only at 150 mg/kg/day. In the absence of degenerative or necrotic change this was considered an adaptive response to increased metabolic demand. At 150 and 450 mg/kg/day kidney histopathology (higher incidence and severity of acidophilic globules in the tubular epithelium) and increased kidney weights observed in male rats only are suggestive of male rat specific alpha-2-microglobulin nephropathy, which was subsequently confirmed. There were no adverse effects on other organs including the reproductive organs. The fertility of male rats given an average oral dose of 148 to 190 mg/day of hexylene glycol for 130 days was unchanged when compared to that of the control group. Developmental study in rats found increased pup mortality and reduced body weight gain at 1000 mg/kg/day. Hexylene glycol produced negative Ames test results for Salmonella Typhimurium TA 1535, TA 1537, TA 1538, TA 98, TA 100 with and without activation. ECOTOXICITY STUDIES: Aquatic toxicity was evaluated in the sea urchin embryo (Arbacia punctulata) by the inhibition of tritiated thymidine incorporation after a brief exposure to toxic chemicals. In preliminary trials, using compounds including hexylene glycol, results accurately predicted reduced survival and morphological delay after 48 hr exposures.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
该物质可以通过吸入其气溶胶被吸收进入人体。
The substance can be absorbed into the body by inhalation of its aerosol.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,吞食,皮肤和/或眼睛接触
inhalation, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、皮肤、呼吸系统刺激;头痛、眩晕、恶心、不协调,中枢神经系统抑制;皮炎,皮肤敏感化。
irritation eyes, skin, respiratory system; headache, dizziness, nausea, incoordination, central nervous system depression; dermatitis, skin sensitization
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
喉咙痛。咳嗽。
Sore throat. Cough.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
... Mice /were fed hexylene glycol/ orally 20 mg/day in 2 mL of whole milk for up to 81 days ... . approximately 40% of the hexylene glycol was accounted for in the urine, but only 4% of the amount excreted was free glycol; the other 36% was conjugated with glycuronic acid.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
不容易通过皮肤吸收……。
... Not readily absorbed through the skin ... .
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
以尿液排出,部分(20-25%)以结合形式。
Eliminated in urine, partly (20-25%) in conjugated forms.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
口服己二醇给大鼠和兔子,导致血浆和尿液中的己糖酸含量显著增加。
... Oral administration of hexylene glycol to rats & rabbits resulted in a substantial increase in the amt of hexuronates in the plasma & in the urine.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露限值:Ceiling: 25 ppm (125 mg/m3)
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/38
-
WGK Germany:1
-
海关编码:2905399090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:SA0810000
-
包装等级:Z01
-
危险性防范说明:P501,P270,P264,P280,P302+P352,P337+P313,P305+P351+P338,P362+P364,P332+P313,P301+P312+P330
-
危险性描述:H302,H315,H319
-
储存条件:储存于阴凉、通风的库房。远离火种、热源,并与氧化剂、还原剂、酸类及食用化学品分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 己二醇;2-甲基-2,4-戊二醇 |
| 化学品英文名称: | Hexalene glycol;2-Methyl-2,4-pentadiol |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 107-41-5 |
| 分子式: | C 6 H 14 O 2 |
| 分子量: | 118.17 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:己二醇;2-甲基-2,4-戊二醇 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 高浓度对眼、呼吸道有刺激性。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃,有毒,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用大量流动清水彻底冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水或生理盐水冲洗。就医。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。 |
| 食入: | 给饮足量温水,催吐,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或与氧化剂接触,有引起燃烧的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 雾状水、泡沫、二氧化碳、干粉、砂土。 |
| 消防员的个体防护: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 121 |
| 自燃温度(℃): | 无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源。防止流入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。也可以用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、还原剂、酸类接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、还原剂、酸类、食用化学品分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:未制订标准美国TLV—TWA:未制订标准美国TLVWN:ACGIH 121mg/m3 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 高浓度接触时,应该佩戴防毒面具。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 必要时戴防化学品手套。 |
| 其他防护: | 工作现场严禁吸烟。避免长期反复接触。定期体检。注意个人清洁卫生。 |
| 第九部分:理化特性 |
| 外观与性状: | 略带臭味的液体。 |
| pH: | |
| 熔点(℃): | -40 |
| 沸点(℃): | 197.1 |
| 相对密度(水=1): | 0.92(20℃) |
| 相对蒸气密度(空气=1): | 4.1 |
| 饱和蒸气压(kPa): | 0.007(20℃) |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 121 |
| 引燃温度(℃): | 无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 6 H 14 O 2 |
| 分子量: | 118.17 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 与水混溶,可混溶于乙醇,溶于多数有机溶剂。 |
| 主要用途: | 用于有机合成。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强酸、强氧化剂、强还原剂、酰基氯、酸酐。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:400mg/kg(大鼠经口);1299mg/kg(小鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、还原剂、酸类、食用化学品等混装混运。运输车船必须彻底清洗、消毒,否则不得装运其它物品。船运时,配装位置应远离卧室、厨房,并与机舱、电源、火源等部位隔离。公路运输时要按规定路线行驶。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
描述
2-甲基-2,4-戊二醇(英文名:2-Methyl-2,4-pentanediol,简称MPD)是一种有机物二醇类化合物,含有一个手性碳原子。常温下为无色液体,可由二丙酮醇氢化制得。该物质具有温和的甜味,并且与水混溶,能溶于乙醇及其他多数有机溶剂。
性质与稳定性- 应避免与强酸、强氧化剂、强还原剂、酰基氯及酸酐接触。2-甲基-2,4-戊二醇为可燃性液体,容易吸湿但对金属无腐蚀性。它类似于乙二醇在碱中稳定,即使煮沸也不分解,但在有酸存在时易与醛发生缩合反应,生成1,3-二噁烷的衍生物。
- 该物质存在于白肋烟的烟叶及烟气中。
2-甲基-2,4-戊二醇是一种多功能精细化工产品,广泛应用于农药、生化工程、感光材料和合成香料等领域。此外,它还能用作高级有机溶剂,在金属表面处理剂生产中作为除锈除油的添加剂;用于纺织助剂、涂料与乳胶漆里;以及在化妆品中用作农药稳定剂或日化保湿剂、香精香料原料、液压油、高温润滑油、刹车油、干洗剂、印刷油墨、颜料分散剂和木材防腐剂等方面,还可以作为渗透剂和防冻剂。
合成方法2-甲基-2,4-戊二醇的主要合成方法有两种:一种是从2,4,4,6-四甲基–1,3-二氧环己烷的甲醇分解制得;另一种则是以丙酮缩合物二丙酮醇为原料进行加氢反应。后者工艺简单,原料易得。
化学性质该物质为无色液体,熔点 -40℃,沸点 198℃(1.33kPa),相对密度 0.9216(20/4℃),折光率 1.4276。闪点 93℃。它能溶于水、醇、醚及低级脂肪烃,并具有轻微的甜香味。
用途该物质可用作溶剂、香料、医用消毒剂、织物处理助剂和造纸与皮革加工助剂等。大鼠口服LD50值为4700毫克/公斤。此外,它还用作农药稳定剂及柴机油防冻剂等。
合成方法2-甲基-2,4-戊二醇由丙酮缩合得到双丙酮醇后经液相加氢而制得。
安全信息- 类别:易燃液体。
- 毒性分级:中毒。急性口服毒性(大鼠)LD50: 3700 毫克/公斤;(小鼠)LD50: 3097 毫克/公斤。
-
刺激数据:
- 皮肤反应(兔子):465毫克,轻度
- 眼睛反应(兔子):93毫克,重度
- 可燃性危险特性:易燃,燃烧时产生刺激烟雾。
- 储运特性:库房应通风、低温干燥保存。
- 灭火剂:干粉、泡沫、砂土及水均可使用。
-
职业标准:
- 时间加权平均浓度(TWA):125毫克/立方米
- 短时间暴露限值(STEL):190毫克/立方米
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (R)-(-)-2-甲基-2,4-戊二醇 2-methyl-2,4-pentanediol 99210-90-9 C6H14O2 118.176 弱酸性宝石红5BL optically active 2-methyl-2,4-pentanediol 99210-91-0 C6H14O2 118.176 2-甲基-2-戊醇 2-methylpentan-2-ol 590-36-3 C6H14O 102.177 4-甲基-2-戊醇 4-methyl-2-pentanol 108-11-2 C6H14O 102.177 —— 1,1-dimethyl-3-hydroxybutyl hydroperoxide 66734-30-3 C6H14O3 134.175 —— 2-Methyl-2,4-dimethoxypentan 41223-32-9 C8H18O2 146.23
反应信息
-
作为反应物:描述:参考文献:名称:Bartleson; Burk; Lankelma, Journal of the American Chemical Society, 1946, vol. 68, p. 2517摘要:DOI:
-
作为产物:参考文献:名称:在室温下使用N-甲苯磺酰基-4-氯苯磺酰亚氨基氟化物(SulfoxFluor)对醇进行快速脱氧氟化摘要:醇类的脱氧氟化是获取烷基氟化物的最基本的方法,因此,迫切需要开发出耐贮存,易处理,氟经济且高选择性的脱氧氟化试剂。这项工作描述了结晶化合物N的发展甲苯磺酰基-4-氯苯磺酰亚胺基氟(SulfoxFluor),作为一种新型的脱氧氟化剂,具有上述所有优点,在脱氧氟化领域是罕见的。由于具有磺酰亚胺基的多维调节能力,SulfoxFluor的氟化速率优于2-吡啶磺酰氟(PyFluor),并且在氟经济方面也优于全氟丁烷磺酰氟(PBSF)。它与醇的反应不仅可以耐受各种功能,包括受空间位阻更大的醇羟基,而且还具有很高的氟化/消除选择性。由于SulfoxFluor可以轻松地由廉价的材料制成,并且无需特殊技术即可安全地进行处理,DOI:10.1002/chem.201901176
-
作为试剂:描述:参考文献:名称:Scalable process for the preparation of a rapamycin 42-ester from a rapamycin 42-ester boronate摘要:提供了一种可扩展的过程,通过将雷帕霉素42-酯硼酸酯与二醇反应,并通过再结晶和与二醇处理来纯化粗雷帕霉素42-酯。还提供了一种从含有丙酮和雷帕霉素杂质的母液中分离和纯化雷帕霉素42-酯硼酸酯的方法。公开号:US20070129541A1
文献信息
-
Additives and products including oligoesters申请人:——公开号:US20030199593A1公开(公告)日:2003-10-23The present invention relates to oligoesters and their use or the creation of additives. Oligoester containing additives and/or oligoesters themselves may be used for formulating pharmaceutical preparations, cosmetics or personal care products such as shampoos and conditioners. These oligoesters are particularly useful for the creation of multi-purpose additives that can impart conditioning, long substantivity and/or UV protection. Individual oligoesters and oligoester mixtures are described.本发明涉及寡酯及其用途或添加剂的制备。含有寡酯的添加剂和/或寡酯本身可用于配制药物制剂、化妆品或个人护理产品,如洗发水和护发素。这些寡酯对于制备能够赋予调理、长效性和/或紫外线保护的多功能添加剂特别有用。描述了单独的寡酯和寡酯混合物。
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
[EN] ANTIBACTERIAL COMPOUNDS<br/>[FR] COMPOSÉS ANTIBACTÉRIENS申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2019199979A1公开(公告)日:2019-10-17The present application provides compounds of formula: Methods of using these compounds for killing bacterial growth and treating bacterial infections are also provided.本申请提供了以下化合物的公式:还提供了使用这些化合物杀灭细菌生长和治疗细菌感染的方法。
-
[EN] HERBICIDALLY ACTIVE HETEROARYL-S?BSTIT?TED CYCLIC DIONES OR DERIVATIVES THEREOF<br/>[FR] DIONES CYCLIQUES SUBSTITUÉES PAR HÉTÉROARYLE À ACTIVITÉ HERBICIDE OU DÉRIVÉS DE CELLES-CI申请人:SYNGENTA LTD公开号:WO2011012862A1公开(公告)日:2011-02-03The invention relates to a compound of formula (I), which is suitable for use as a herbicide wherein G is hydrogen or an agriculturally acceptable metal, sulfonium, ammonium or latentiating group; Q is a unsubstituted or substituted C3-C8 saturated or mono-unsaturated heterocyclyl containing at least one heteroatom selected from O, N and S, or Q is heteroaryl or substituted heteroaryl; m is 1, 2 or 3; and Het is an optionally substituted monocyclic or bicyclic heteroaromatic ring; and wherein the compound is optionally an agronomically acceptable salt thereof.
表征谱图
-
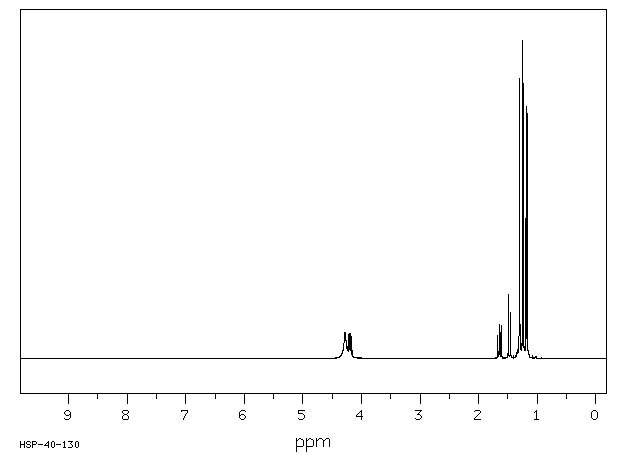
氢谱1HNMR
-
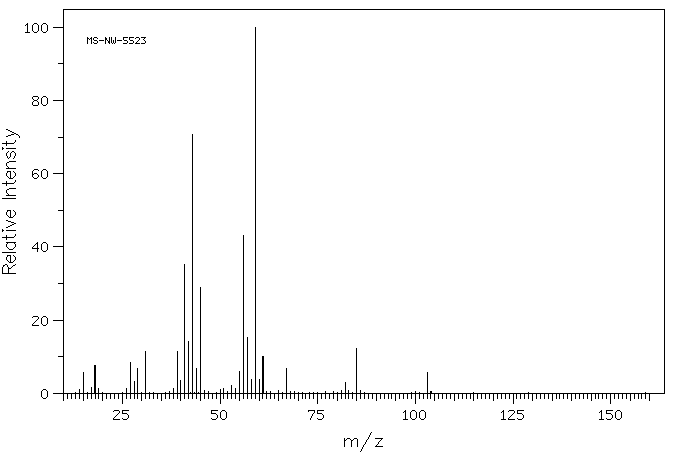
质谱MS
-
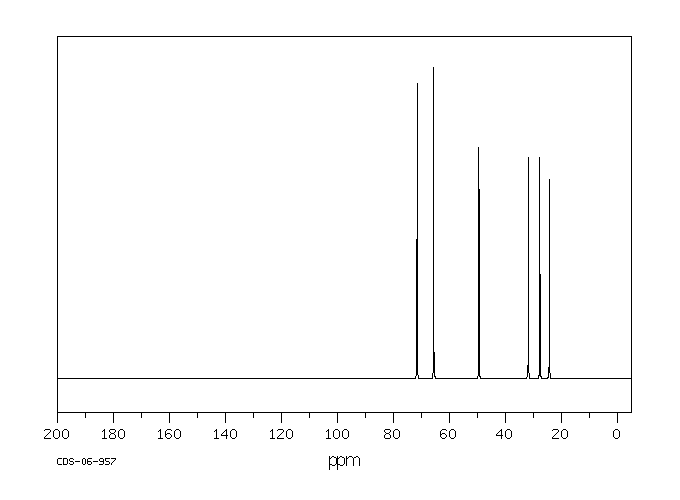
碳谱13CNMR
-
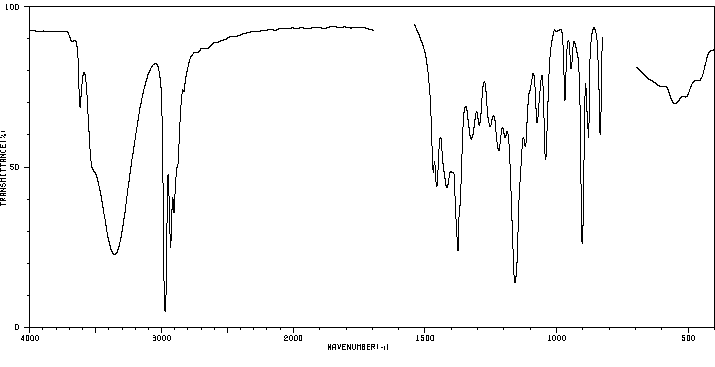
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷