弱酸性宝石红5BL | 99210-91-0
中文名称
弱酸性宝石红5BL
中文别名
——
英文名称
optically active 2-methyl-2,4-pentanediol
英文别名
(4S)-2-Methyl-2,4-pentanediol;(4S)-2-methylpentane-2,4-diol
CAS
99210-91-0;12220-29-0
化学式
C6H14O2
mdl
——
分子量
118.176
InChiKey
SVTBMSDMJJWYQN-YFKPBYRVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
制备方法与用途
用途: 弱酸性酱红 5BL 用于羊毛、蚕丝和锦纶的染色及其织物的直接印花。 也可用于羊毛、蚕丝和锦纶织物的染色与印花。
生产方法: 以间氨基苯磺酸、1-萘胺和 N,N-二羟乙基苯胺为原料,首先将间氨基苯磺酸重氮化,再与 1-萘胺偶合;接着进行第二次重氮化,最后与 N,N-二羟乙基苯胺偶合得产物。经过滤、干燥、粉碎后得到成品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-2,4-戊二醇 2-methyl-2,4-pentanediol 107-41-5 C6H14O2 118.176
反应信息
-
作为反应物:描述:弱酸性宝石红5BL 在 caesium carbonate 、 三乙胺 作用下, 以 二氯甲烷 、 二甲基亚砜 为溶剂, 反应 26.0h, 生成 4-(4,6-diamino-3-iodo-1H-pyrazolo[3,4-d]pyrimidin-1-yl)-2-methylpentan-2-ol参考文献:名称:WO2023/31738摘要:公开号:
-
作为产物:描述:二丙酮醇 在 optically active hydrogenation complex catalyst 、 lithium chloride 氢气 作用下, 以 甲醇 为溶剂, 60.0 ℃ 、9.0 MPa 条件下, 反应 12.0h, 以99.1%的产率得到弱酸性宝石红5BL参考文献:名称:WO2006/54644摘要:公开号:
文献信息
-
Absolute Configuration of Secondary Alcohols by <sup>1</sup>H NMR: In Situ Complexation of α-Methoxyphenylacetic Acid Esters with Barium(II)作者:Rosa García、José M. Seco、Saulo A. Vázquez、Emilio Quiñoá、Ricardo RigueraDOI:10.1021/jo0256989日期:2002.6.1allows the assignment of the absolute configuration of chiral secondary alcohols by NMR using only one derivative is presented. All that is needed is (a) the derivatization of the alcohol of unknown configuration with one enantiomer--either the (R)- or the (S)--of alpha-methoxyphenylacetic acid (MPA), (b) the recording of the 1H NMR spectrum of the resulting ester in MeCN-d3, and (c) addition of a barium(II)提出了一种新颖的方法,该方法允许仅使用一种衍生物通过NMR分配手性仲醇的绝对构型。所需要做的只是(a)用一种对映体(α-甲氧基苯基乙酸(MPA)的(R)-或(S))衍生化未知构型的醇,(b)记录在MeCN-d3中所得酯的1H NMR光谱,以及(c)向NMR管中添加钡盐(II)[即Ba(ClO4)2]直至饱和并记录第二光谱。将R / S构型分配给醇需要花费几分钟,并且需要将钡(II)的添加与预期的(R)和(S)对映异构体是根据简化的模型反映的,该模型反映了与钡络合产生的构象变化及其对化学位移的影响。这些构象变化基于实验NMR和CD结果,结果表明与MPA酯形成的钡(II)配合物使顺式(sp)和反平面(ap)形式之间的构象平衡向最稳定的形式移动( sp),并且这导致由醇的某些取代基上的MPA苯基引起的屏蔽作用增加。此外,从头算起,Hartree-Fock(HF)和密度泛函理论(DFT)计算为有关Ba2
-
Upcycling a plastic cup: one-pot synthesis of lactate containing metal organic frameworks from polylactic acid作者:Benjamin Slater、So-On Wong、Andrew Duckworth、Andrew J. P. White、Matthew R. Hill、Bradley P. LadewigDOI:10.1039/c9cc02861g日期:——Waste PLA can be upcycled to metal organic frameworks of potential high value in a one-pot synthesis scheme, where PLA depolymerisation occurs in situ. Three homochiral lactate based frameworks were successfully synthesised and characterised from PLA as a feed source, including ZnBLD. The chiral separation ability of ZnBLD was maintained.
-
“Single nickel source” in situ fabrication of a stable homochiral MOF membrane with chiral resolution properties作者:Zixi Kang、Ming Xue、Lili Fan、Jinying Ding、Lijia Guo、Lianxun Gao、Shilun QiuDOI:10.1039/c3cc42376j日期:——A homochiral MOF membrane was successfully and facilely synthesized using an in situ growth method, which had the advantages of cheap raw materials, simple operation and high thermal stability. A diol isomer mixture was used to test the separation efficiency of the membrane at different temperatures and pressures.使用原位生长法成功且简便地合成了同手性的MOF膜,该方法具有原料便宜、操作简单且热稳定性高的优点。使用二醇异构体混合物在不同温度和压力下测试了膜的分离效率。
-
NMR determination of the absolute configuration of chiral 1,2- and 1,3-diols作者:Hiroki Fukui、Yukiharu Fukushi、Satoshi TaharaDOI:10.1016/s0040-4039(03)00845-1日期:2003.51,2- and 1,3-diols examined was derivatized exclusively to a single diastereomeric acetal by the use of a new axially chiral reagent, 2′-methoxy-1,1′-binaphthalene-8-carbaldehyde (MBC). The absolute configuration of the original 1,2- and 1,3-diols was determined by the NOE correlation between the proton signals of the reagent moiety and those of the diol moiety in the acetals.
-
Resolution of racemic mixtures via lipase catalysis in organic solvents作者:Gerald Kirchner、Mark P. Scollar、Alexander M. KlibanovDOI:10.1021/ja00310a052日期:1985.11
表征谱图
-
氢谱1HNMR
-
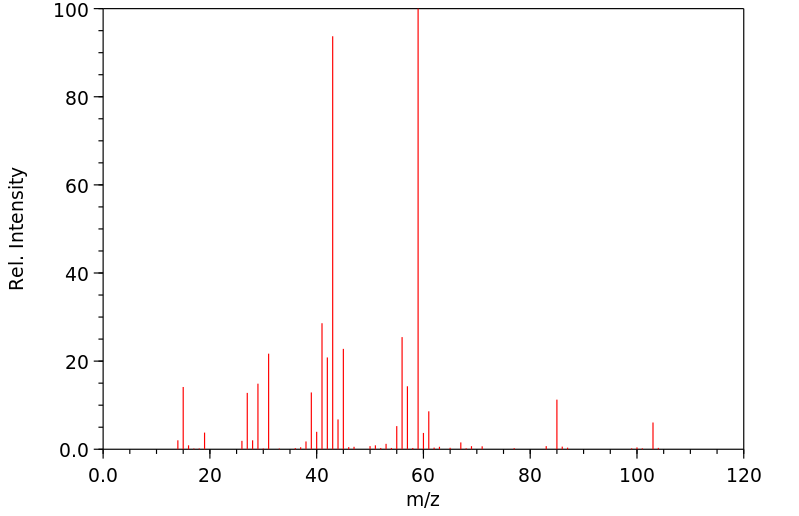
质谱MS
-
碳谱13CNMR
-
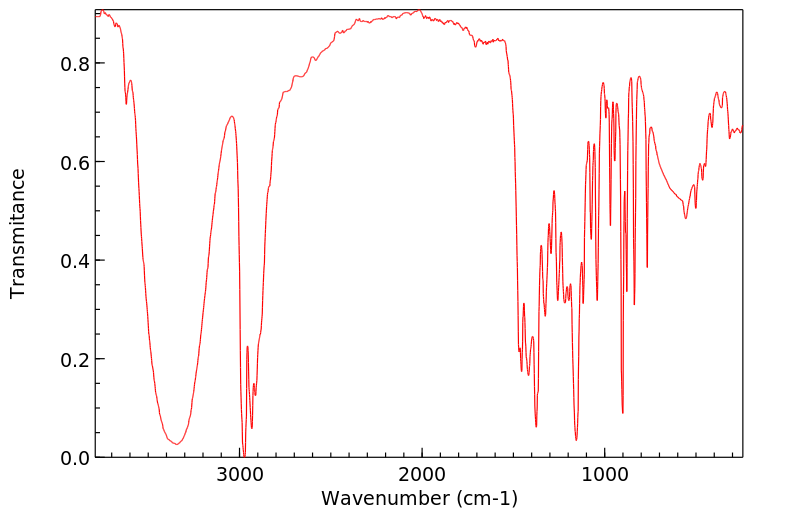
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷