8-氯喹啉 | 611-33-6
中文名称
8-氯喹啉
中文别名
8-氯代喹啉;8-氯喹林
英文名称
8-chloroquinoline
英文别名
——
CAS
611-33-6
化学式
C9H6ClN
mdl
MFCD00047618
分子量
163.606
InChiKey
RUSMDMDNFUYZTM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-20 °C
-
沸点:288.5 °C
-
密度:1.28
-
闪点:>110℃
-
保留指数:1459.6
-
稳定性/保质期:
性质与稳定性:
常温常压下,该物质不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39,S37/39
-
危险类别码:R36/37/38
-
海关编码:2933499090
-
WGK Germany:3
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302+H312+H332,H315,H319,H335
-
储存条件:贮存: 将密器密封,并储存在密封的主容器中,应放置于阴凉、干燥处。
SDS
| Name: | 8-Chloroquinoline 99% (gc) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 611-33-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 611-33-6 | 8-Chloroquinoline | 99.0 | 210-265-6 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Dust may cause mechanical irritation.
Skin:
No information regarding skin irritation and other potential effects was found.
Ingestion:
The toxicological properties of this substance have not been fully investigated. May cause central nervous system effects.
Inhalation:
Inhalation of dust may cause respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 611-33-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear slight yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 288.5 deg C @ 760.00MM HG
Freezing/Melting Point: -20 deg C
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: soluble
Specific Gravity/Density:
Molecular Formula: C9H6ClN
Molecular Weight: 163.61
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 611-33-6: VB2330000 LD50/LC50:
Not available.
Carcinogenicity:
8-Chloroquinoline - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 611-33-6: No information available.
Canada
CAS# 611-33-6 is listed on Canada's NDSL List.
CAS# 611-33-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 611-33-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,8-二氯喹啉 2,8-dichloroquinoline 4470-83-1 C9H5Cl2N 198.051 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,8-二氯喹啉 2,8-dichloroquinoline 4470-83-1 C9H5Cl2N 198.051 8-氯-2-羟基喹啉 8-chloroquinolin-2-ol 23981-25-1 C9H6ClNO 179.606 —— 8-chloro-3-iodoquinoline 847727-21-3 C9H5ClIN 289.503 8-氯喹啉-N-氧化物 8-chloroquinoline-N-oxide 866874-13-7 C9H6ClNO 179.606 8-氯-4-羟基喹啉 4-hydroxy-8-chloroquinoline 57797-97-4 C9H6ClNO 179.606 —— 8-chloro-2-ethynylquinoline 866874-10-4 C11H6ClN 187.628 喹啉 quinoline 91-22-5 C9H7N 129.161 8-氯-2-苯基喹啉 8-chloro-2-phenylquinoline 745064-23-7 C15H10ClN 239.704 —— 8-chloro-1-methylquinolin-2(1H)-one 67805-52-1 C10H8ClNO 193.633 —— 2-methyl-4-[2'-(8'-chloroquinolyl)]-but-3-yn-2-ol 866874-09-1 C14H12ClNO 245.708
反应信息
-
作为反应物:描述:参考文献:名称:光诱导、苯肼促进的芳基氟化物、氯化物、溴化物和碘化物的无过渡金属脱卤摘要:在这项研究中,我们提出了一种直接且高效的芳基卤化物光触发氢化方法,无需过渡金属催化剂。通过光、PhNHNH2 和碱的协同利用,我们成功地引发了所需的自由基介导的氢化过程。值得注意的是,利用温和的反应条件,多种芳基卤化物,包括氟化物、氯化物、溴化物和碘化物,可以选择性地转化为其相应的(杂)芳烃对应物,且产率极高。此外,该方法表现出与不同官能团和杂环化合物的显着相容性,突出了其多功能性和在各种化学转化中使用的潜力。DOI:10.3390/molecules28196915
-
作为产物:描述:2-氯-N-(2-丙炔-1-基)苯胺 在 silver hexafluoroantimonate 作用下, 反应 0.08h, 生成 8-氯喹啉参考文献:名称:微波辅助条件下由芳族硼酸,氨水和炔丙基溴一锅法合成N-芳基炔丙基胺摘要:摘要在微波辅助条件下,由芳族硼酸,氨水和炔丙基溴容易地一锅合成N-芳基炔丙基胺。该反应可通过两步程序在总共10分钟内顺利完成,包括铜催化的芳香族硼酸与氨水的偶联以及随后的炔丙基溴的炔丙基化反应。DOI:10.1016/j.cclet.2014.03.011
文献信息
-
一种含苯并呋喃结构的膦配体及其制备方法 和应用
-
Remote site-selective C–H activation directed by a catalytic bifunctional template作者:Zhipeng Zhang、Keita Tanaka、Jin-Quan YuDOI:10.1038/nature21418日期:2017.3In chemical syntheses, the activation of carbon–hydrogen (C–H) bonds converts them directly into carbon–carbon or carbon–heteroatom bonds without requiring any prior functionalization. C–H activation can thus substantially reduce the number of steps involved in a synthesis. A single specific C–H bond in a substrate can be activated by using a ‘directing’ (usually a functional) group to obtain the desired在化学合成中,碳-氢 (C-H) 键的活化将它们直接转化为碳-碳或碳-杂原子键,无需任何事先的功能化。因此,C-H 活化可以大大减少合成中涉及的步骤数。可以通过使用“定向”(通常是官能团)基团来激活底物中的单个特定 C-H 键,从而选择性地获得所需的产物。由于所讨论的 C-H 键与导向基团的距离以及底物的形状,这种 C-H 活化反应的适用性会受到严重限制,但已经开发了几种方法来克服这些限制。在这样一种方法中,通过使用共价连接的 U 形模板,已经利用对官能团和底物 C-H 键之间的远端和几何关系的理解来实现元选择性 C-H 激活。然而,在没有合适的官能团来连接它的情况下,这个模板的化学计量安装是不可行的。在这里,我们报告了一种催化双功能腈模板的设计,该模板通过可逆配位而不是共价键结合杂环底物。与该模板协调的两个金属中心具有不同的作用:一个可逆地锚定催化剂附近的底物,另一个切割远程 C-H 键。使用这种策略,我们演示了远程、
-
A General Catalyst Based on Cobalt Core–Shell Nanoparticles for the Hydrogenation of N‐Heteroarenes Including Pyridines作者:Kathiravan Murugesan、Vishwas G. Chandrashekhar、Carsten Kreyenschulte、Matthias Beller、Rajenahally V. JagadeeshDOI:10.1002/anie.202004674日期:2020.9.28core–shell based nanoparticles prepared by template synthesis of cobalt‐pyromellitic acid on silica and subsequent pyrolysis. The optimal catalyst material allows for general and selective hydrogenation of pyridines, quinolines, and other heteroarenes including acridine, phenanthroline, naphthyridine, quinoxaline, imidazo[1,2‐a]pyridine, and indole under comparably mild reaction conditions. In addition
-
Catalytic 1,2-Regioselective Dearomatization of N-Heteroaromatics via a Hydroboration作者:Heng Liu、Maxim Khononov、Moris S. EisenDOI:10.1021/acscatal.8b00074日期:2018.4.6catalyzed highly 1,2-regioselective dearomatization of pyridines via a hydroboration process is reported herein. Twelve different kinds of meta- and para-substituted pyridines are applicable to this reaction, giving the corresponding N-boryl-1,2-dihydropyridine products in high yields. Other N-heteroaromatic compounds, such as benzo-fused N-heterocycles, pyrazines, pyrimidines, 1,3,5-triazine, and benzothiazole
-
Iodine catalyzed reduction of quinolines under mild reaction conditions作者:Chun-Hua Yang、Xixi Chen、Huimin Li、Wenbo Wei、Zhantao Yang、Junbiao ChangDOI:10.1039/c8cc04262d日期:——synthetically versatile tetrahydroquinoline molecules with I2 and HBpin is described. In the presence of iodine (20 mol%) as a catalyst, reduction of quinolines and other N-heteroarenes proceeded readily with hydroboranes as the reducing reagents. The broad functional-group tolerance, good yields and mild reaction conditions imply high practical utility.
表征谱图
-
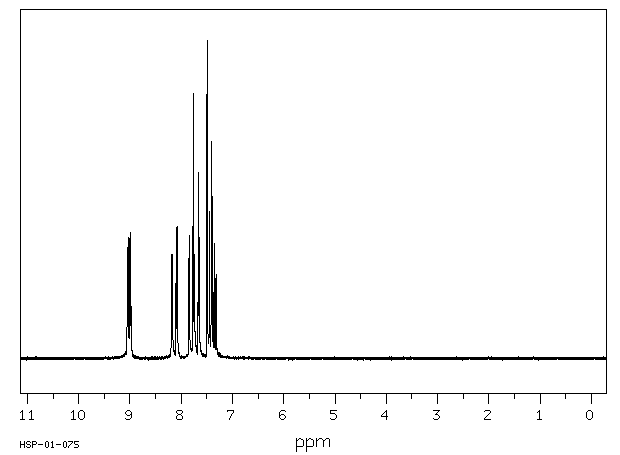
氢谱1HNMR
-
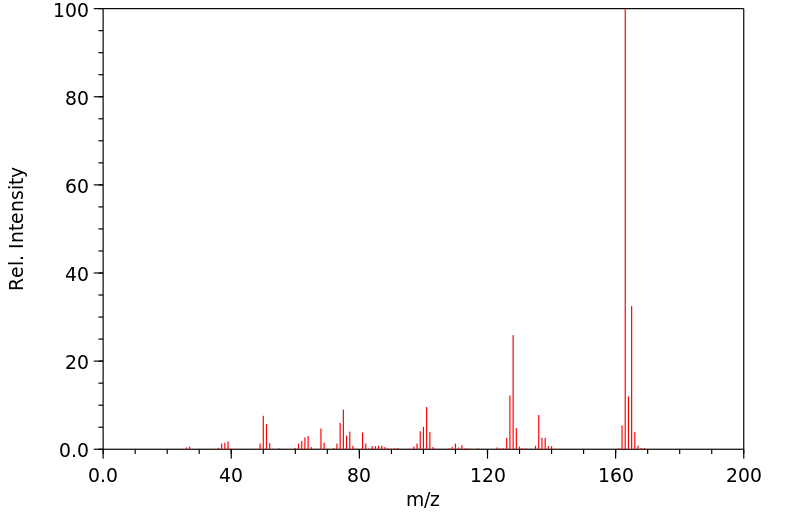
质谱MS
-
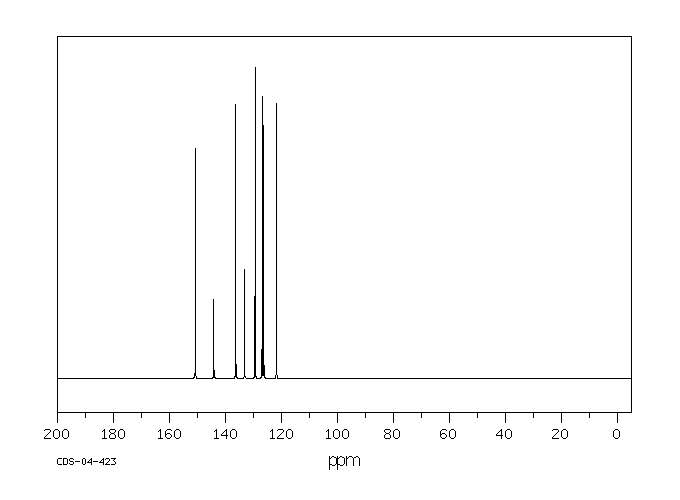
碳谱13CNMR
-
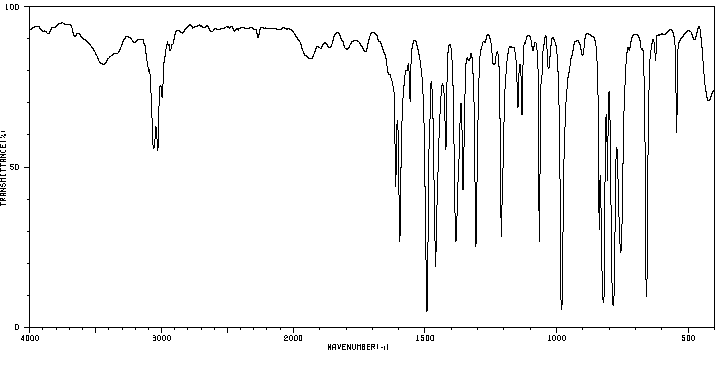
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43