2-氧代戊烷-1,5-二甲酸二乙酯 | 5965-53-7
中文名称
2-氧代戊烷-1,5-二甲酸二乙酯
中文别名
2-氧代戊二酸-1,5-二乙酯;二乙基2-氧代戊二酸酯
英文名称
Diethyl 2-ketoglutarate
英文别名
diethyl 2-oxoglutarate;Diethyl 2-oxopentanedioate
CAS
5965-53-7
化学式
C9H14O5
mdl
MFCD00032382
分子量
202.207
InChiKey
NGRAIMFUWGFAEM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:126-128°C 12mm
-
密度:0,98 g/cm3
-
闪点:>113°C
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:14
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:69.7
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2918300090
-
安全说明:S24/25
-
WGK Germany:3
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:储存条件:室温下干燥密封保存。
SDS
| Name: | Diethyl oxalpropionate 97% Material Safety Data Sheet |
| Synonym: | Diethyl 2-methyl-2'-oxosuccinat |
| CAS: | 5965-53-7 |
Synonym:Diethyl 2-methyl-2'-oxosuccinat
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5965-53-7 | Diethyl oxalpropionate | 97 | 227-750-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5965-53-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear very slight yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 138 deg C @ 23.00 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0730 g/cm3
Molecular Formula: C9H14O5
Molecular Weight: 202.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Acids, bases, oxidizing agents, reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5965-53-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diethyl oxalpropionate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 5965-53-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5965-53-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5965-53-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备具体步骤如下:在500毫升的三口瓶中加入250毫升的乙醇,室温下依次加入43.8克(0.3摩尔,1.0当量)2-氧代戊二酸和22克Amberlyst 15(湿型,离子交换树脂)。加完后搅拌反应1小时,通过薄层色谱(TLC)跟踪监测反应至结束。将反应液经过过滤并浓缩处理后得到2-氧代戊烷-1,5-二甲酸二乙酯57.8克,收率约为95%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 alpha-酮戊二酸 α-ketoglutaric acid 328-50-7 C5H6O5 146.1 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-oxoglutaric acid 1-ethyl ester 35818-60-1 C7H10O5 174.153 alpha-酮戊二酸 α-ketoglutaric acid 328-50-7 C5H6O5 146.1
反应信息
-
作为反应物:描述:2-氧代戊烷-1,5-二甲酸二乙酯 在 盐酸 作用下, 反应 2.0h, 以72%的产率得到alpha-酮戊二酸参考文献:名称:Cappon, J. J.; Baart, J.; Walle, G. A. M. van der, Recueil des Travaux Chimiques des Pays-Bas, 1991, vol. 110, # 5, p. 158 - 166摘要:DOI:
-
作为产物:描述:参考文献:名称:7-fused 2-(piperazinoalkyl) indole derivatives, intermediates and摘要:具有抗过敏性能的药理活性化合物对应于公式I ##STR1##,该化合物可以在苯环中单取代或双取代,并描述了它们的酸盐和/或含有硫的化合物的S-单取代物或二氧化物,以及用于它们制备的过程和中间体。公开号:US05324725A1
文献信息
-
Oxidation of hydrazones by hypervalent organoiodine reagents: Regeneration of the carbonyl group and facile syntheses of α-acetoxy and α-alkoxy azo compounds作者:Derek H.R. Barton、Joseph Cs. Jaszberenyi、Wansheng Liu、Tetsuro ShinadaDOI:10.1016/0040-4020(96)00940-4日期:1996.11derivatives of α-keto esters were prepared. The carbonyl group was readily regenerated in high yield from phenylhydrazones through oxidative hydrolysis using hypervalent organoiodine(III) reagents—either bis(trifluoroacetoxy)-iodobenzene (BTIB) in aqueous acetonitrile or hydroxy(tosyloxy)iodobenzene (HTIB) in chloroform. α-Acetoxy phenyl- or methylazo compounds were readily synthesized by oxidation of the
-
Radical Alkylations of Alkyl Halides and Unactivated C-H Bonds Using Vinyl Triflates作者:Sunggak Kim、Jin Lee、Kyoung-Chan Lim、Xiangjian MengDOI:10.1055/s-0029-1219960日期:2010.7Radical alkylations of activated alkyl iodides and bromides were achieved using vinyl triflates in the presence of hexadimethyltin, whereas those of unactivated C-H bonds using vinyl triflates proceeded cleanly under tin-free conditions.
-
[EN] PRODRUGS OF ALPHA-KETOGLUTARATE, ALPHA-KETOBUTYRATE, ALPHA-KETOISOVALERATE, AND ALPHA-KETOISOHEXANOATE, AND USES THEREOF<br/>[FR] PROMÉDICAMENTS D'ALPHA-CÉTOGLUTARATE, D'ALPHA-CÉTOBUTYRATE, D'ALPHA-CÉTOISOVALÉRATE, ET D'ALPHA-CÉTOISOHEXANOATE, ET LEURS UTILISATIONS申请人:UNIV CALIFORNIA公开号:WO2020081836A1公开(公告)日:2020-04-23The present disclosure provides compounds and compositions capable of extending lifespan, and methods of use thereof.本公开提供了能够延长寿命的化合物和组合物,以及它们的使用方法。
-
Identification and Structure–Activity Relationship of HDAC6 Zinc-Finger Ubiquitin Binding Domain Inhibitors作者:Renato Ferreira de Freitas、Rachel J. Harding、Ivan Franzoni、Mani Ravichandran、Mandeep K. Mann、Hui Ouyang、Mark Lautens、Vijayaratnam Santhakumar、Cheryl H. Arrowsmith、Matthieu SchapiraDOI:10.1021/acs.jmedchem.8b00258日期:2018.5.24combination therapy with proteasome inhibitors in multiple myeloma. Pharmacologically displacing ubiquitin from the zinc-finger ubiquitin-binding domain (ZnF-UBD) of HDAC6 is an underexplored alternative to catalytic inhibition. Here, we present the discovery of an HDAC6 ZnF-UBD-focused chemical series and its progression from virtual screening hits to low micromolar inhibitors. A carboxylate mimicking the
-
Simultaneous monitoring of the bioconversion from lysine to glutaric acid by ethyl chloroformate derivatization and gas chromatography-mass spectrometry作者:Yeong-Hoon Han、Tae-Rim Choi、Ye-Lim Park、Hun-Suk Song、Yong-Keun Choi、Hyun-Joong Kim、Shashi Kant Bhatia、Ranjit Gurav、Kyungmoon Park、See-Hyoung Park、Wooseong Kim、Yung-Hun YangDOI:10.1016/j.ab.2020.113688日期:2020.5a gas chromatography - mass spectrometry (GC-MS) method with ethyl chloroformate (ECF) derivatization to simultaneously monitor the major metabolites. We determined the suitability of GC-MS analysis using defined concentrations of six metabolites (l-lysine, cadaverine, 5-aminovaleric acid, 2-oxoglutaric acid, glutamate, and glutaric acid) and their mass chromatograms, regression equations, regression戊二酸是增塑剂的前体,可用于生产聚酯酰胺,酯增塑剂,缓蚀剂等。戊二酸可以通过生物转化或化学合成产生,并且在反应过程中产生一些代谢产物和中间体。为确保反应效率,底物,中间体和产物,尤其是生物转化系统中的底物,中间体和产物,应受到密切监控。迄今为止,尽管高效液相色谱(HPLC)需要单独进行耗时的衍生化和非衍生化分析,但通常已用于分析戊二酸相关的代谢产物。为了代替这种不合理的分析方法,我们在本文中采用了氯甲酸乙酯(ECF)衍生化的气相色谱-质谱(GC-MS)方法来同时监测主要代谢物。我们使用六种代谢物(1-赖氨酸,尸胺,5-氨基戊酸,2-氧戊二酸,谷氨酸和戊二酸)的规定浓度及其质谱图,回归方程式,回归系数值( R2),动态范围(mM)和保留时间(RT)。该方法成功地监测了复杂发酵液的生产过程。回归方程式,回归系数值(R2),动态范围(mM)和保留时间(RT)。该方法成功地监测了复杂发酵液的生产过
表征谱图
-
氢谱1HNMR
-
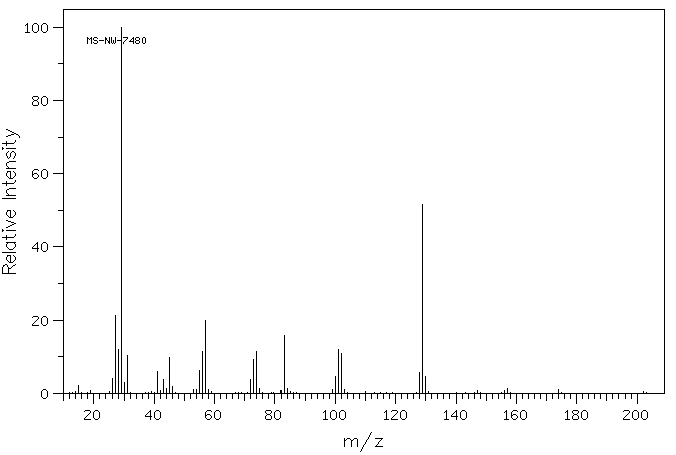
质谱MS
-
碳谱13CNMR
-
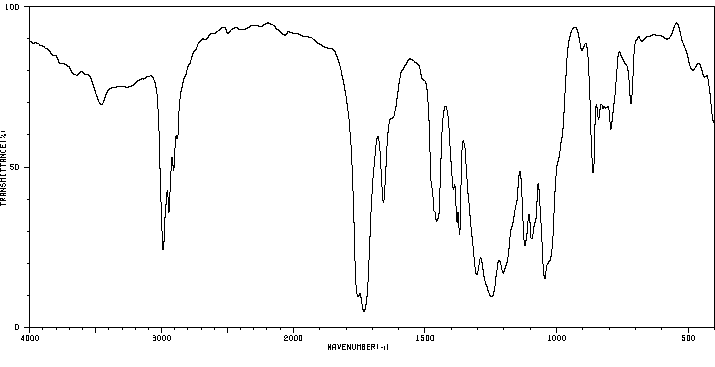
红外IR
-
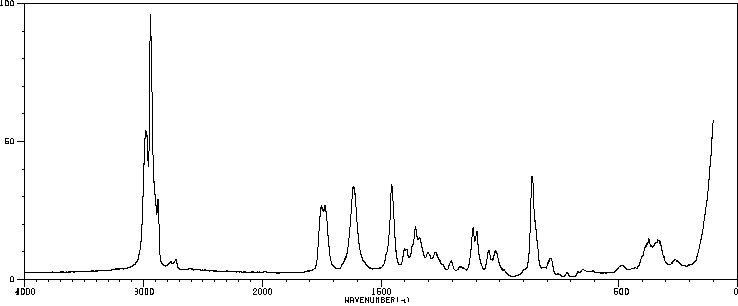
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰基乙酸
顺-3-己烯-1-丙酮酸
青霉酸
钠氟草酰乙酸二乙酯
醚化物
酮霉素
辛酸,2,4-二羰基-,乙基酯
草酸乙酯钠盐
草酰乙酸二乙酯钠盐
草酰乙酸二乙酯
草酰乙酸
草酰丙酸二乙酯
苯乙酰丙二酸二乙酯
苯丁酸,b-羰基-,2-丙烯基酯
聚氧化乙烯
羟基-(3-羟基-2,3-二氧代丙基)-氧代鏻
磷酸二氢2-{(E)-2-[4-(二乙胺基)-2-甲基苯基]乙烯基}-1,3,3-三甲基-3H-吲哚正离子
碘化镝
硬脂酰乙酸乙酯
甲氧基乙酸乙酯
甲氧基乙酰乙酸酯
甲基氧代琥珀酸二甲盐
甲基4-环己基-3-氧代丁酸酯
甲基4-氯-3-氧代戊酸酯
甲基4-氧代癸酸酯
甲基4-氧代月桂酸酯
甲基4-(甲氧基-甲基磷酰)-2,2,4-三甲基-3-氧代戊酸酯
甲基3-羰基-2-丙酰戊酸酯
甲基3-氧代十五烷酸酯
甲基2-氟-3-氧戊酯
甲基2-氟-3-氧代己酸酯
甲基2-氟-3-氧代丁酸酯
甲基2-乙酰基环丙烷羧酸酯
甲基2-乙酰基-4-甲基-4-戊烯酸酯
甲基2-乙酰基-2-丙-2-烯基戊-4-烯酸酯
甲基2,5-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代丁酸酯
甲基1-异丁酰基环戊烷羧酸酯
甲基1-乙酰基环戊烷羧酸酯
甲基1-乙酰基环丙烷羧酸酯
甲基1-乙酰基-2-乙基环丙烷羧酸酯
甲基(2Z,4E,6E)-2-乙酰基-7-(二甲基氨基)-2,4,6-庚三烯酸酯
甲基(2S)-2-甲基-4-氧代戊酸酯
甲基(1S,2R)-2-乙酰基环丙烷羧酸酯
甲基(1R,2R)-2-乙酰基环丙烷羧酸酯
瑞舒伐他汀杂质
瑞舒伐他汀杂质
环氧乙烷基甲基乙酰乙酸酯
环戊戊烯酸,Β-氧代,乙酯