2-(氯甲基)-5-甲氧基吡喃-4-酮 | 40838-34-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118 °C
-
沸点:317.5±42.0 °C(Predicted)
-
密度:1.30±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (2-氯甲基)-5-羟基-4H-吡喃-4-酮 chlorokojic acid 7559-81-1 C6H5ClO3 160.557 2-(羟基甲基)-5-甲氧基-4H-吡喃-4-酮 2-Hydroxymethyl-5-methoxy-4H-pyran-4-on 6269-25-6 C7H8O4 156.138 5-(苄氧基-2-(羟甲基)-4H-吡喃-4-酮 5-benzyloxy-2-hydroxymethyl-4H-pyran-4-one 15771-06-9 C13H12O4 232.236 曲酸 5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one 501-30-4 C6H6O4 142.111 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl allomaltol 6266-91-7 C7H8O3 140.139 2-(羟基甲基)-5-甲氧基-4H-吡喃-4-酮 2-Hydroxymethyl-5-methoxy-4H-pyran-4-on 6269-25-6 C7H8O4 156.138 5-甲氧基-4H-吡喃-4-酮-2-甲醛 5-methoxy-4-oxo-4H-pyran-2-carbaldehyde 35438-43-8 C7H6O4 154.122 —— 2-Azidomethyl-5-methoxy-4H-pyran-4-on 134563-17-0 C7H7N3O3 181.151 —— (5-Methoxy-4-oxo-4H-2-yl)butylcarbamate 139108-81-9 C12H17NO5 255.271
反应信息
-
作为反应物:描述:2-(氯甲基)-5-甲氧基吡喃-4-酮 在 sodium azide 、 氢溴酸 、 potassium carbonate 、 溶剂黄146 、 苯酚 作用下, 以 N,N-二甲基甲酰胺 、 丙酮 为溶剂, 反应 30.5h, 生成 (5-Methoxy-4-oxo-4H-2-yl)butylcarbamate参考文献:名称:Cizmaricova; Miskikova; Gregan, Pharmazie, 1991, vol. 46, # 6, p. 460 - 460摘要:DOI:
-
作为产物:描述:参考文献:名称:新的白藜芦醇类似物作为改进的生物活性结构:设计、合成和计算建模摘要:[显示省略]DOI:10.1016/j.bioorg.2023.106965
文献信息
-
A NEW PEPTIDE DEFORMYLASE INHIBITOR COMPOUND AND MANUFACTURING PROCESS THEREOF申请人:KANG Jae Hoon公开号:US20100168421A1公开(公告)日:2010-07-01The present invention relates to the novel antibacterial compounds having potent antibacterial activity as inhibitors of peptide deformylase. This invention further relates to pharmaceutically acceptable salts thereof, to processes for their preparation, and to pharmaceutical compositions containing them as an active ingredient.本发明涉及具有强效抗菌活性的新型抗菌化合物,作为肽变形酶抑制剂。该发明还涉及其药用盐,其制备方法,以及含有它们作为活性成分的药物组合物。
-
Studies on Tyrosinase Inhibitory and Antioxidant Activities of Benzoic Acid Derivatives Containing Kojic Acid Moiety作者:Ho-Sik Rho、Chang-Seok Lee、Soo-Mi Ahn、Yong-Deog Hong、Song-Seok Shin、Young-Ho Park、Soo-Nam ParkDOI:10.5012/bkcs.2011.32.12.4411日期:2011.12.20Although compound 2 has a kojic acid moiety for chelation to copper in the active site of tyrosinase, tyrosinase inhibitory activity is not found. We also synthesized a benzoate ester of kojic acid without an adamantane moiety and investigated its tyrosinase inhibitory and radical scavenging activities. Scheme 1 shows the synthetic pathways for the benzoate ester of kojic acid. The reaction of kojic acid酪氨酸酶 1 广泛分布于微生物、动物和植物中,负责动物黑色素化和植物褐变。黑色素对皮肤的光损伤起着至关重要的保护作用。然而,异常黑色素色素沉着的产生是人类严重的审美问题。在食品工业中,酪氨酸酶负责收获后处理和加工过程中受损水果的酶促褐变反应。因此,一直致力于开发可用作皮肤美白剂2或保鲜食品防腐剂3的酪氨酸酶抑制剂。曲酸 4 (1) 由多种真菌和细菌产生,因其酪氨酸酶抑制活性而被广泛用作皮肤美白剂。由于其自由基清除活性,曲酸还被证明可以防止光损伤。5 然而,它的抑制活性和储存性能不足以用于化妆品和作为食品的抗褐变剂。为了增加其活性,已合成了许多半合成曲酸衍生物。许多这些化合物是通过修饰 C-2 羟基形成酯、6 个醚、7 个硫化物 8 和肽 9 衍生物而形成的,因为 C-5 烯醇羟基被认为是抑制酪氨酸酶的药效团。最近,我们合成了一种含有金刚烷部分的曲酸 2,4-二羟基苯甲酸酯 (2),并评估了它的酪氨酸酶活性。10
-
Discovery of 4-(Benzylaminomethylene)isoquinoline-1,3-(2<i>H</i>,4<i>H</i>)-diones and 4-[(Pyridylmethyl)aminomethylene]isoquinoline-1,3-(2<i>H</i>,4<i>H</i>)-diones as Potent and Selective Inhibitors of the Cyclin-Dependent Kinase 4作者:Hwei-Ru Tsou、Xiaoxiang Liu、Gary Birnberg、Joshua Kaplan、Mercy Otteng、Tritin Tran、Kristina Kutterer、Zhilian Tang、Ron Suayan、Arie Zask、Malini Ravi、Angela Bretz、Mary Grillo、John P. McGinnis、Sridhar K. Rabindran、Semiramis Ayral-Kaloustian、Tarek S. MansourDOI:10.1021/jm801026e日期:2009.4.23oline-1,3-(2H,4H)-dione derivatives reported here represents a novel class of potential antitumor agents, which potently and selectively inhibit CDK4 over CDK2 and CDK1. In the benzylamino headpiece, a 3-OH substituent is required on the phenyl ring for CDK4 inhibitory activity, which is further enhanced when an iodo, aryl, heteroaryl, t-butyl, or cyclopentyl substituent is introduced at the C-6 position此处报道了一系列4-(苄基氨基亚甲基)异喹啉-1,3-(2 H,4 H)-二酮和4-[(吡啶基甲基)氨基亚甲基]异喹啉-1,3-(2 H,4 H)-二酮衍生物代表一类新型的潜在抗肿瘤药,与CDK2和CDK1相比,它有力和选择性地抑制CDK4。在苄基氨基机头,一个3-OH的取代基是必需的苯环为CDK4抑制活性,这是进一步提高上时碘,芳基,杂芳基,吨在异喹啉-1,3-二酮核的C-6位置引入丁基或环戊基取代基。为了规避与酚OH基团的4 -取代的3-OH苯基机头有关的代谢责任,我们采取两种方法:第一,引入氮气ø -或p -在苯环的3-OH基团; 第二,用N-取代的2-吡啶酮代替苯基头基。我们在这里介绍了合成,SAR数据,代谢稳定性数据和CDK4模拟模型,该模型解释了我们的CDK4选择性抑制剂的结合,效能和选择性。
-
Ethers and thioethers of kojic acid and preparation thereof申请人:RHONE POULENC SA公开号:US02865930A1公开(公告)日:1958-12-23
The invention comprises compounds of the formula <;FORM:0781413/IV (a)/1>; where R is alkyl, X is O or NH, Y is O or S, and Ar is aryl, which may have one or more alkyl, alkoxy, hydroxyalkyl, nitro or halogen substituents; the alkyl and alkoxy groups have not more than 4 carbon atoms. The compounds are prepared (1) by the action of H-Y-Ar on the appropriate pyrones or pyridones having a -CH2Z substituent where Z is a reactive ester group such as halide, sulphuric or sulphonic ester, preferably in a solvent at 50-100 DEG C. in the presence of a basic condensing agent, or (2) by the alkylation of the corresponding 5-hydroxy compounds. In addition the pyridones may be made from the pyrones by heating with ammonia. Examples show the preparation by method (1) of 2-aryloxymethyl-5 - methoxy - 4 - pyrones where aryl group is phenyl, b -naphthyl, and the following substituted phenyl groups:-chloro (3 isomers), 2:4-dichloro, 2:4:6-trichloro, pentachloro, methyl (3 isomers), dimethyl (6 isomers), 2:4:6-trimethyl, 2-methyl-4- and -6-chloro, 3-methyl-4-chloro, 4-methyl-2-chloro, 2-methyl-4:6-dichloro, 4 - methyl - 2:6 - dichloro, 2:4 - dimethyl-6-chloro, 3:5-dimethyl-4-chloro, 4-isopropyl, 4-cyclohexyl, 2- and 4-nitro, 2-methylol, 2:4 - dichloro - 6 - methylol, 2 - chloro - 4:6 - dimethylol, 4 - chloro - 2:6 - dimethylol, 4 - methyl - 2 - methylol, 4 - methyl - 2:6 - dimethylol, 2:5 - dimethyl - 4 - methylol, 3:4 - dimethyl - 6 - methylol, 3 - methoxy, and 2 - methoxy-4-, -5- and -6-methyl; also 2-(21-methylolphenoxymethyl) - 5 - ethoxy - 4 - pyrone and 2-(41-chlorophenylthiomethyl) - 5 - methoxy-4-pyrone. I further examples 2-(41 chlorophenoxymethyl) - 5 - methoxy - 4 - pyridone (hydrochloride described) and 2-(21-methyl - 61 - chlorophenoxymethyl) - 5 - methoxy-4-pyridone are made from the corresponding pyrones and ammonia. It is also stated that Ar may also be ethylphenyl, ethoxyphenyl or hydroxy-ethyl-phenyl.ALSO:Compositions for use as plant growth regulants contain as active ingredient a compound of the formula: <;FORM:0781413/I/1>; where R is alkyl, X is O or NH, Y is O or S and Ar is aryl which may have one or more alkyl, alkoxy, hydroxyalkyl, nitro or halogen substituents; the alkyl and alkoxy groups have not more than 4 carbon atoms. The compositions may be in the form of powders, sprays, aerosols, emulsions or solutions in organic or aqueous organic solvents. There may also be present wetting agents, synergists and other plant growth regulants. In an example 2-(21 : 41-dichlorophenoxymethyl)-5-methoxy-4-pyrone is dissolved in dimethylformamide and diluted with water to give a solution which enhances root formation when plant stems are soaked with it. Many other suitable compounds are mentioned.ALSO:Compositions for use as systemic fungicides or herbicides contain as essential ingredient a compound of the formula <;FORM:0781413/VI/1>; where R is alkyl, X is O or NH, Y is O or S and Ar is aryl which may have one or more alkyl, alkoxy, hydroxyalkyl, nitro or halogen substituents; the alkyl and alkoxy groups have not more than 4 carbon atoms. The compositions may be in the form of powders, sprays, aerosols, emulsions or solutions in organic or aqueous organic solvents. There may also be present wetting agents, synergists, and other plant-growth regulants or fungicides. In examples, fungicides are made of (1) 2-(21-methyl - 41 - chlorophenoxymethyl) - 5 - methoxy-4-pyrone dispersed in water with the aid of a wetting agent; and (2) 2-(31:51-dimethylphenoxy - methyl) - 5 - methoxy - 4 - pyrone and talc similarly dispersed; herbicides are made from (3) 2-(2-(21:41-dichlorophenoxymethyl) - 5 - methoxy - 4 - pyrone; and (4) 2-(41 - chlorophenoxymethyl) - 5 - methoxy - 4-pyrone, in each case dissolved in toluene and acetone and dispersed in water with the aid of a wetting agent. Many other suitable compounds are mentioned.
该发明包括以下公式的化合物:其中R为烷基,X为O或NH,Y为O或S,Ar为芳基,可能具有一个或多个烷基、烷氧基、羟基烷基、硝基或卤素取代基;烷基和烷氧基基团的碳原子数不超过4。这些化合物通过以下方法制备:(1)在适当的吡喃酮或吡啶酮上作用H-Y-Ar,其中吡喃酮或吡啶酮具有一个-CH2Z取代基,其中Z是反应性酯基,如卤化物、硫酸酯或磺酸酯,最好在50-100摄氏度的溶剂中,在碱性缩合剂的存在下进行,或者(2)通过对应的5-羟基化合物的烷基化制备。此外,吡啶酮可以通过与氨加热制备。示例表明,通过方法(1)制备了2-芳氧基甲基-5-甲氧基-4-吡喃,其中芳基为苯基、β-萘基,以及以下取代苯基:氯(3个异构体)、2,4-二氯、2,4,6-三氯、五氯、甲基(3个异构体)、二甲基(6个异构体)、2,4,6-三甲基、2-甲基-4-和-6-氯、3-甲基-4-氯、4-甲基-2-氯、2-甲基-4,6-二氯、4-甲基-2,6-二氯、2,4-二甲基-6-氯、3,5-二甲基-4-氯、4-异丙基、4-环己基、2-和4-硝基、2-甲基醇、2,4-二氯-6-甲基醇、2-氯-4,6-二甲基醇、4-氯-2,6-二甲基醇、4-甲基-2-甲基醇、4-甲基-2,6-二甲基醇、2,5-二甲基-4-甲基醇、3,4-二甲基-6-甲基醇、3-甲氧基、2-甲氧基-4-, -5-和-6-甲基;还有2-(21-甲基醇苯氧基甲基)-5-乙氧基-4-吡喃和2-(41-氯苯硫醚基甲基)-5-甲氧基-4-吡喃。另外还有一些其他化合物的例子。 -
Inhibitory activity of novel kojic acid derivative containing trolox moiety on melanogenesis作者:Soo Mi Ahn、Ho Sik Rho、Heung Soo Baek、Yung Hyup Joo、Yong Deog Hong、Song Seok Shin、Young-Ho Park、Soo Nam ParkDOI:10.1016/j.bmcl.2011.09.122日期:2011.12A novel kojic acid derivative containing a trolox moiety, (±)-5-hydroxy-4-oxo-4H-pyran-2-yl methyl 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylate (3a), was synthesized. The two biologically active compounds, namely, kojic acid and trolox, were conjugated via an ester bond as they are expected to behave synergistically. The antioxidant activity and the tyrosinase inhibitory activity of this novel一种新型的曲酸衍生物,其含有trolox部分,(±)-5-羟基-4-氧代-4 H-吡喃-2-基甲基6-羟基-2,5,7,8-四甲基苯并-2-羧酸酯(3a),已合成。两种生物活性化合物,即曲酸和trolox,通过酯键共轭,因为它们预期具有协同作用。评价了该新型曲酸衍生物对黑素生成的抗氧化活性和酪氨酸酶抑制活性。化合物3a表现出强大的酪氨酸酶抑制活性和自由基清除活性。有限的结构-活性关系(SAR)研究表明,酪氨酸酶抑制活性可能起源于曲酸部分,自由基清除活性可能是由于trolox的酚羟基所致。在基于细胞的测定中,化合物3a还表现出有效的脱色活性。有限的SAR研究表明,3a的脱色活性可能是由于曲酸和其trolox部分的协同活性。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
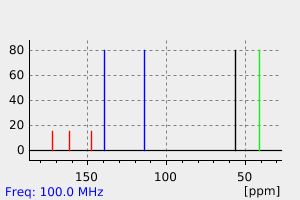
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息