9-溴蒽 | 1564-64-3
中文名称
9-溴蒽
中文别名
9-溴代蒽
英文名称
9-Bromoanthracene
英文别名
9-bromoanthracen
CAS
1564-64-3
化学式
C14H9Br
mdl
MFCD00001243
分子量
257.129
InChiKey
ZIRVQSRSPDUEOJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:97-100 °C (lit.)
-
沸点:303.85°C (rough estimate)
-
密度:1.4251 (rough estimate)
-
溶解度:可溶于氯仿(少许)、DMSO(少许)、甲醇(少许)
-
保留指数:2125;2140;2125;2143
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:29049090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:0-6°C下应密封储存。
SDS
| Name: | 9-Bromoanthracene 96% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1564-64-3 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1564-64-3 | 9-Bromoanthracene | 96% | 216-359-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1564-64-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 97 - 100 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H9Br
Molecular Weight: 256.967
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1564-64-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
9-Bromoanthracene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1564-64-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1564-64-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1564-64-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
9-溴蒽
简介
9-溴蒽是一种性能优良的荧光、磷光发光材料,广泛应用于光学、化妆品、电子、半导体和电致发光材料等领域。
用途 理化性质9-溴蒽为白色结晶。熔点65-66℃,沸点190℃(0.16kPa,升华),相对密度1.409。该物质溶于乙酸和二硫化碳。
合成方法在氩气保护下,将1.8 mmol PIFA(1当量)加入到12 mL干燥的二氯甲烷中,然后向溶液中滴加3.30 mmol 三甲基溴硅烷(2.0当量)。此时可以看到澄清溶液变为澄清橙色。随后,在另一反应管中加入1.7 mmol 蒽,并在室温下进行反应。反应结束后,将体系倒入水中并用二氯甲烷萃取,蒸发有机溶剂并在高真空下充分干燥浓缩油状物即可得到目标产物分子9-溴蒽。
上下游信息
反应信息
-
作为反应物:参考文献:名称:在水-氯仿介质中用溴化钾将取代的多环芳烃间接电化学氧化为相应的对醌摘要:已经通过使用Pt阳极以恒定电流密度(40mA / cm 2)电解溴化钾(3.0M)的水溶液,间接电化学合成了一系列取代的蒽和萘的醌衍生物。如物理和光谱数据所证实,这些反应导致相应的对苯醌的产率良好至优异。DOI:10.1016/j.tetlet.2014.08.121
-
作为产物:描述:参考文献:名称:灵敏的TICT探针具有比例荧光特性,可检测溶液和气相中的肼。摘要:设计并合成了基于分子内电荷转移(TICT)的双探针双氰基乙烯基-9-苯基蒽(DPA),用于检测肼(N2H4),且检出限良好(LOD,7.85 nM(0.25 ppb))。在与肼相互作用时,吸电子的双氰基乙烯基的末端官能改变为给电子的氨基/ hydr官能。因此,探针的光物理性质的显着变化归因于电荷传播方向的变化。带有肼的探针在介质中显示出比例荧光“开启”响应以及肉眼敏感的颜色变化。表面形态学研究(SEM和TEM)分别表明了探针DPA和衍生物DPA-HDz的非晶性和晶体性质。探针的导电行为在与肼相互作用时降低,这是因为基质的无定形性降低并且相对更刚性的晶体结构增加。此外,该探针还用于检测溶液中和试纸条上的肼蒸气,并具有良好的肉眼敏感响应。DOI:10.1016/j.saa.2020.118153
-
作为试剂:参考文献:名称:[EN] OXIDATION OF AROMATIC HYDROCARBONS USING BROMINATED ANTHRACENE PROMOTERS
[FR] OXYDATION D'HYDROCARBURES AROMATIQUES AU MOYEN DE PROMOTEURS A L'ANTHRACENE BROME摘要:公开号:WO2005000779A3
文献信息
-
PHOTOSENSITIVE RESIN COMPOSITION, OXIME SULFONATE COMPOUND, METHOD FOR FORMING CURED FILM, CURED FILM, ORGANIC EL DISPLAY DEVICE, AND LIQUID CRYSTAL DISPLAY DEVICE申请人:FUJIFILM Corporation公开号:US20130171415A1公开(公告)日:2013-07-04Disclosed is a photosensitive resin composition comprising: (Component A) an oxime sulfonate compound represented by Formula (1); (Component B) a resin comprising a constituent unit having an acid-decomposable group that is decomposed by an acid to form a carboxyl group or a phenolic hydroxy group; and (Component C) a solvent wherein in Formula (1) R 1 denotes an alkyl group, an aryl group, or a heteroaryl group, each R 2 independently denotes a hydrogen atom, an alkyl group, an aryl group, or a halogen atom, Ar 1 denotes an o-arylene group or an o-heteroarylene group, X denotes O or S, and n denotes 1 or 2, provided that of two or more R 2 s present in the compound, at least one denotes an alkyl group, an aryl group, or a halogen atom.
-
ORGANIC COMPOUND AND ORGANIC LIGHT EMITTING DEVICE USING THE SAME申请人:Kim Kong Kyeom公开号:US20140077166A1公开(公告)日:2014-03-20The present invention provides an organic light emitting device comprising a first electrode, at least one organic layer and a second electrode, laminated successively, in which at least one layer of the organic layer has a polycyclic aromatic hydrocarbon as a core and comprises at least one of a derivative in which a substituted or unsubstituted C 2-30 cycloalkane, or a substituted or unsubstituted C 5-50 polycycloalkane is directly fused to the core or fused to a substituent of the core: and a new organic compound usable in the organic light emitting device. Furthermore, the present invention provides a charge carrier extracting, injecting or transporting material which has a polycyclic aromatic hydrocarbon as a core and comprises a derivative in which a substituted or unsubstituted C 2-30 cycloalkane, or a substituted or unsubstituted C 5-50 polycycloalkane is directly fused to the core or fused to a substituent of the core.
-
Aromatic substitution in ball mills: formation of aryl chlorides and bromides using potassium peroxomonosulfate and NaX作者:Robert Schmidt、Achim Stolle、Bernd OndruschkaDOI:10.1039/c2gc16508b日期:——Aryl chlorides and bromides are formed from arenes in a ball mill using KHSO5 and NaX (X = Cl, Br) as oxidant and halogen source, respectively. Investigation of the reaction parameters identified operating frequency, milling time, and the number of milling balls as the main influencing variables, as these determine the amount of energy provided to the reaction system. Assessment of liquid-assisted grinding conditions revealed, that the addition of solvents has no advantageous effect in this special case. Preferably activated arenes are halogenated, whereby bromination afforded higher product yields than chlorination. Most often reactions are regio- and chemoselective, since p-substitution was preferred and concurring side-chain oxidation of alkylated arenes by KHSO5 was not observed.
-
一种9,10位取代的蒽的制备及纯化方法
-
ORGANIC ELECTROLUMINESCENCE DEVICE AND ANTHRACENE DERIVATIVE申请人:IKEDA Hidetsugu公开号:US20110034744A1公开(公告)日:2011-02-10An anthracene derivative having a specific asymmetric structure is provided. The asymmetric anthracenes are useful in an organic electroluminescence device and exhibit efficient light emission and a long performance lifetime.
表征谱图
-
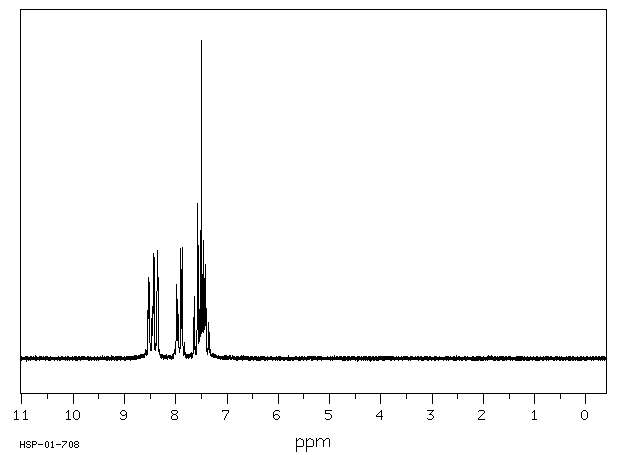
氢谱1HNMR
-
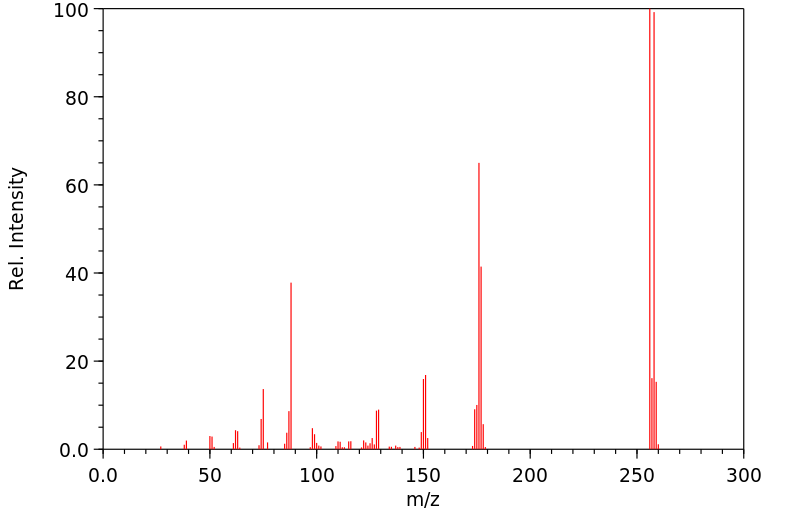
质谱MS
-
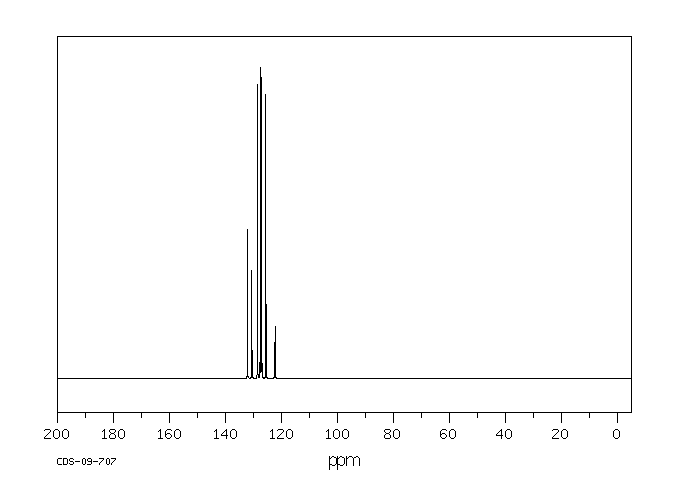
碳谱13CNMR
-
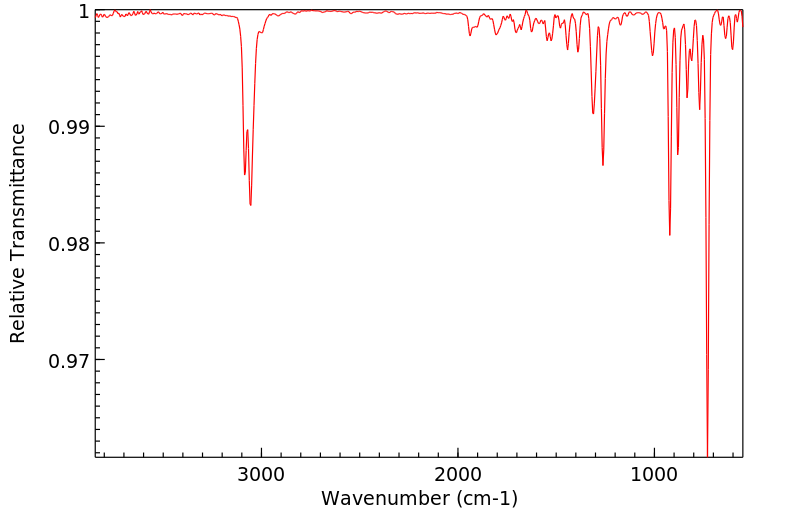
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62