5,6-二甲氧基-8-硝基喹啉 | 5333-02-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:77.2
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2933499090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-甲氧基-8-硝基喹啉 6-methoxy-8-nitroquinoline 85-81-4 C10H8N2O3 204.185 5-氯-6-甲氧基-8-硝基喹啉 5-chloro-6-methoxy-8-nitro-quinoline 64992-86-5 C10H7ClN2O3 238.63 —— 5-bromo-6-methoxy-8-nitroquinoline 5347-15-9 C10H7BrN2O3 283.081 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-甲氧基-8-硝基-1H-喹啉-5-酮 5-Hydroxy-6-methoxy-8-nitro-chinolin 5323-58-0 C10H8N2O4 220.185 —— 2-isopropyl-5,6-dimethoxy-8-nitroquinoline 685092-58-4 C14H16N2O4 276.292 —— 2-tert-butyl-5,6-dimethoxy-8-nitroquinoline 685092-59-5 C15H18N2O4 290.319 —— 8-amino-5,6-dimethoxyquinoline 6938-02-9 C11H12N2O2 204.228 —— 2-isopropyl-5,6-dimethoxy-8-quinolinamine 685092-67-5 C14H18N2O2 246.309 —— 5,6-dimethoxy-8-[4-amino-1-methylbutylamino]quinoline 47136-26-5 C16H23N3O2 289.378 —— 2-tert-butyl-5,6-dimethoxy-8-quinolinamine 685092-68-6 C15H20N2O2 260.336 5-氯-6-甲氧基-8-硝基喹啉 5-chloro-6-methoxy-8-nitro-quinoline 64992-86-5 C10H7ClN2O3 238.63 —— N4,N4-diethyl-N1-(5,6-dimethoxy-[8]quinolyl)-1-methyl-butanediyldiamine 110053-16-2 C20H31N3O2 345.485 8-(5-氨基戊烷-2-基氨基)-6-甲氧基-1H-喹啉-5-酮 5-hydroxyprimaquine 57695-07-5 C15H21N3O2 275.351 —— 5-hydroxy-6-demethylprimaquine 87321-06-0 C14H19N3O2 261.324 —— 2-[4-(2-isopropyl-5,6-dimethoxy-8-quinolinamino)pentyl]-1,3-isoindolinedione 685092-76-6 C27H31N3O4 461.561 —— 2-[4-(2-tert-butyl-5,6-dimethoxy-8-quinolinamino)pentyl]-1,3-isoindolinedione 685092-77-7 C28H33N3O4 475.588 - 1
- 2
反应信息
-
作为反应物:描述:5,6-二甲氧基-8-硝基喹啉 在 叔丁基过氧化氢 、 碘 作用下, 以 水 、 乙腈 为溶剂, 反应 24.0h, 以82%的产率得到3-iodo-5,6-dimethoxy-8-nitroquinoline参考文献:名称:Metal-free synthesis of N-fused heterocyclic iodides via C–H functionalization mediated by tert-butylhydroperoxide摘要:直接、区域选择性和无金属合成杂环碘化物的报道。DOI:10.1039/c5cc04013b
-
作为产物:描述:参考文献:名称:Further Syntheses of Primaquine Analogs1摘要:DOI:10.1021/ja01623a039
文献信息
-
Process for production of 8-NHR quinolines申请人:The United States of America as represented by the Secretary of the Army公开号:US04167638A1公开(公告)日:1979-09-11An improved process for producing 8-NHR quinolines from 8-aminoquinolines disclosed. The process comprises reacting 8-aminoquinolines with a substituted alkyl halide in the presence of an amine having a boiling point of 80.degree.-90.degree. C. The amine functions as an acid acceptor whereby the amine salt formed may be efficiently separated from the 8-NHR quinoline formed without expensive or time consuming purification steps. The reaction may be carried out in the presence of a solvent such as an alcohol.
-
Process for preparation of ring-substituted 8-aminoquinoline analogs as antimalarial agents申请人:——公开号:US20040192724A1公开(公告)日:2004-09-30The present invention is concerned with the development of novel 8-aminoquinoline analogs in the treatment and prevention of malaria and the said compound has broad spectrum of activity against the blood as well as tissue stages of the human malaria parasites makes these compounds very attractive in the cure and prevention of malaria caused by drug-sensitive and multidrug resistant strains and also it is expected that development of these compounds as ideal antimalarial agents may lead to suppression as well as radical cure of the malaria infection with single drug therapy.
-
Antimalarial activity of primaquine operates via a two-step biochemical relay作者:Grazia Camarda、Piyaporn Jirawatcharadech、Richard S. Priestley、Ahmed Saif、Sandra March、Michael H. L. Wong、Suet Leung、Alex B. Miller、David A. Baker、Pietro Alano、Mark J. I. Paine、Sangeeta N. Bhatia、Paul M. O’Neill、Stephen A. Ward、Giancarlo A. BiaginiDOI:10.1038/s41467-019-11239-0日期:——
Abstract Primaquine (PQ) is an essential antimalarial drug but despite being developed over 70 years ago, its mode of action is unclear. Here, we demonstrate that hydroxylated-PQ metabolites (OH-PQm) are responsible for efficacy against liver and sexual transmission stages of
Plasmodium falciparum . The antimalarial activity of PQ against liver stages depends on host CYP2D6 status, whilst OH-PQm display direct, CYP2D6-independent, activity. PQ requires hepatic metabolism to exert activity against gametocyte stages. OH-PQm exert modest antimalarial efficacy against parasite gametocytes; however, potency is enhanced ca.1000 fold in the presence of cytochrome P450 NADPH:oxidoreductase (CPR) from the liver and bone marrow. Enhancement of OH-PQm efficacy is due to the direct reduction of quinoneimine metabolites by CPR with the concomitant and excessive generation of H2O2, leading to parasite killing. This detailed understanding of the mechanism paves the way to rationally re-designed 8-aminoquinolines with improved pharmacological profiles.摘要: 盐酸伯氨喹(PQ)是一种重要的抗疟药,但尽管已经开发了70多年,其作用方式仍不清楚。在这里,我们证明羟基化的PQ代谢物(OH-PQm)对疟原虫 的肝和性传播阶段的疗效负责。PQ对肝阶段的抗疟活性取决于宿主CYP2D6状态,而OH-PQm显示出直接的、独立于CYP2D6的活性。PQ需要肝代谢才能对配子体阶段产生活性。OH-PQm对疟原虫配子体的抗疟功效较小,但在肝和骨髓的细胞色素P450 NADPH:氧化还原酶(CPR)的存在下,其效力增强约1000倍。OH-PQm功效的增强是由于CPR对醌亚胺代谢物的直接还原,同时产生大量的H2O2,导致疟原虫死亡。这种机制的详细理解为有理重设计具有改进药理特性的8-氨基喹啉铺平了道路。 -
Elderfield et al., Journal of the American Chemical Society, 1946, vol. 68, p. 1583作者:Elderfield et al.DOI:——日期:——
-
The Skraup Reaction with Acrolein and its Derivatives<sup>1</sup>作者:Harry L. Yale、Jack BernsteinDOI:10.1021/ja01181a075日期:1948.1
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
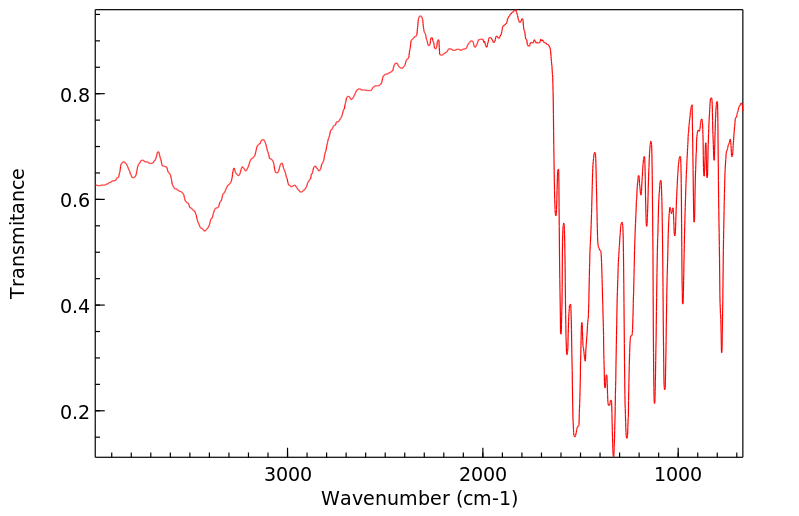
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息