代谢
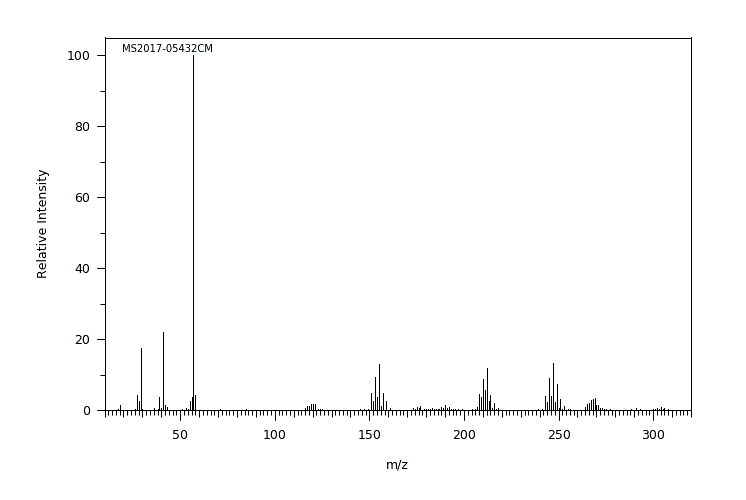
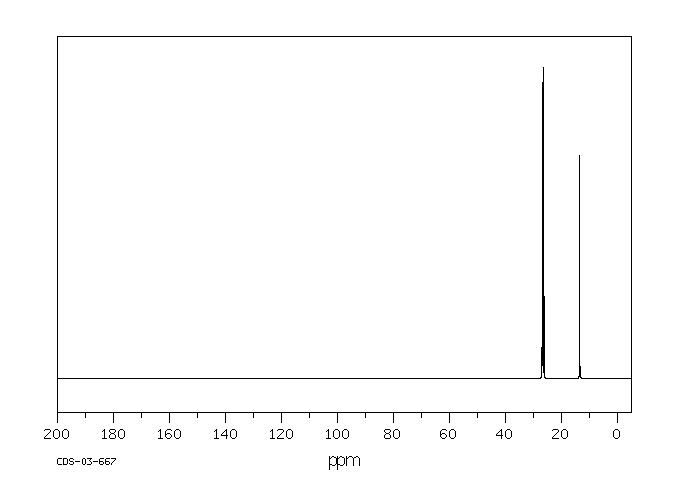
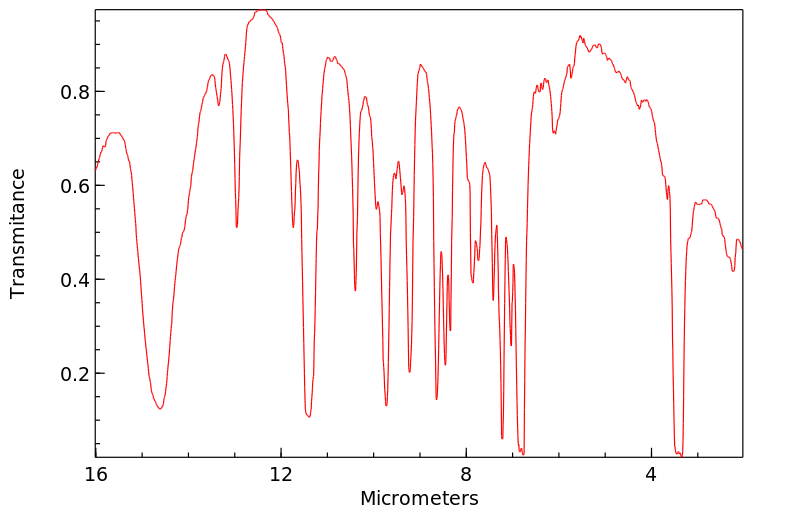
尽管锡金属的吸收非常差,但锡化合物可以通过口服、吸入或皮肤途径被吸收,有机锡化合物的吸收速度远比无机锡化合物快。锡可能进入血液并绑定到血红蛋白上,在体内主要分布并在肾脏、肝脏、肺和骨骼中积累。有机锡化合物可能在肝脏中通过细胞色素P-450酶催化发生脱烷基化、羟基化、脱芳香化和氧化。脱烷基化产生的烷基产物与谷胱甘肽结合并进一步代谢成巯基尿酸衍生物。锡及其代谢物主要通过尿液和粪便排出体外。(L308)
Though tin metal is very poorly absorbed, tin compounds may be absorbed via oral, inhalation, or dermal routes, with organotin compounds being much more readily absorbed than inorganic tin compounds. Tin may enter the bloodstream and bind to hemoglobin, where it is distributed and accumulates mainly in the kidney, liver, lung, and bone. Organotin compounds may undergo dealkylation, hydroxylation, dearylation, and oxidation catalyzed by cytochrome P-450 enzymes in the liver. The alkyl products of dealkylation are conjugated with glutathione and further metabolized to mercapturic acid derivatives. Tin and its metabolites are excreted mainly in the urine and feces. (L308)
来源:Toxin and Toxin Target Database (T3DB)