1,2-O-isopropylidene-α-D-glucofuranurono-6,3-lactone | 20513-98-8
中文名称
——
中文别名
——
英文名称
1,2-O-isopropylidene-α-D-glucofuranurono-6,3-lactone
英文别名
1,2-O-isopropylidene-β-L-idofuranurono-6,3-lactone;1,2-di-O-isopropylidene-α-D-glucofuranurono-6,3-lactone;D-Glucurono-6,3-lactone acetonide;(1S,2R,6R,8R,9R)-9-hydroxy-4,4-dimethyl-3,5,7,11-tetraoxatricyclo[6.3.0.02,6]undecan-10-one
CAS
20513-98-8
化学式
C9H12O6
mdl
——
分子量
216.191
InChiKey
BDBGJSXZKMTMGP-QOHYDMMQSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:119-121°C
-
沸点:386.0±42.0 °C(Predicted)
-
密度:1.410±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)、水(少许)
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:74.2
-
氢给体数:1
-
氢受体数:6
安全信息
-
安全说明:S24/25
-
海关编码:29322090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— D-glucurono-3,6-lactone acetonide 20513-98-8 C9H12O6 216.191 —— D-glucuronolactone 487-44-5 C6H8O6 176.126 —— α-D-glucurono-6,3-lactone —— C6H8O6 176.126 —— α-D-glucurono-γ-lactone 14362-29-9 C6H8O6 176.126 —— D-mannofuranurono-6,3-lactone 575-64-4 C6H8O6 176.126 1,2-O-异亚丙基-D-呋喃葡萄糖 1,2-O-isopropylidene-α-D-glucofuranose 18549-40-1 C9H16O6 220.222 1,2-O-异亚丙基-beta-L-艾杜呋喃糖 1,2-O-isopropylidene-β-L-idofuranose 29747-91-9 C9H16O6 220.222 —— 1,2-O-isopropylidene-5-O-trifluoromethanesulphonyl-α-D-glucuronolactone —— C10H11F3O8S 348.254 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-O-(tert-Butyldimethylsilyl)-1,2-O-isopropyliden-β-D-idofuranurono-6,3-lacton 136632-69-4 C15H26O6Si 330.453 1,2-O-异亚丙基-beta-L-艾杜呋喃糖 1,2-O-isopropylidene-β-L-idofuranose 29747-91-9 C9H16O6 220.222 —— 5,6-anhydro-1,2-O-isopropylidene-β-L-idofuranose 4118-60-9 C9H14O5 202.207 —— 1,2-O-isopropylidene-5-deoxy-α-D-glucofuranose 7057-09-2 C9H16O5 204.223 —— 1,2-O-isopropylidene-N-(prop-2-ynyl)-α-D-glucofuranuronamide 655233-93-5 C12H17NO6 271.27 —— 5-azido-5-deoxy-1,2-O-isopropylidene-α-D-glucuronolactone 66592-81-2 C9H11N3O5 241.203 —— 5-(N-benzyloxycarbonyl)amino-5-deoxy-1,2-O-isopropylidene-α-D-glucuronolactone 54612-59-8 C17H19NO7 349.34 —— 5,6-anhydro-5,6-epithio-1,2-O-isopropylidene-α-D-glucofuranose 37614-79-2 C9H14O4S 218.274 —— 5-azido-5-deoxy-1,2-O-isopropylidene-α-D-glucofuranose 69465-27-6 C9H15N3O5 245.235
反应信息
-
作为反应物:描述:1,2-O-isopropylidene-α-D-glucofuranurono-6,3-lactone 在 acidic cationen-exchanger 、 水 作用下, 生成 L-艾杜糖醛酸钠参考文献:名称:Synthesis1 of L-Iduronic Acid and an Improved Production1 of D-Glucose-6-C14摘要:DOI:10.1021/ja01614a072
-
作为产物:描述:D-glucurono-3,6-lactone acetonide 在 吡啶 、 三氟甲磺酸酐 、 sodium 2,2,2-trifluoroacetate 作用下, 以 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 3.0h, 生成 1,2-O-isopropylidene-α-D-glucofuranurono-6,3-lactone参考文献:名称:合成的1-Deoxynojirimycin N取代肽为N-连接的糖加工提供了更长的中断时间摘要:合成了一组1-脱氧野oji霉素(DNJ)N-连接的肽。他们在体外针对α-葡萄糖苷酶I和II的IC 50值进行了测量,发现两种同工酶的IC 50值都在微摩尔范围内,并且优于针对α-葡萄糖苷酶II的亚氨基糖N B-DNJ(miglustat,3)。基于细胞的研究表明,尽管游离亚氨基糖3在短期孵育(一天)中能最有效地破坏N-连接的聚糖加工,但是当基于细胞的研究延长至三天时,DNJ N-连接的四肽KDEL ,这是一个内质网(ER)保留序列,表现远好于3。在低抑制剂洗脱研究中,NB-DNJ抑制作用在24小时后降至零,但DNJ-KDEL保留了13%的活性。这种方法为将药物靶向ER并延长其活性提供了一种通用方法。此外,它是模块化的,因此,当发现新的效力增强的亚氨基糖时,可以将它们添加到此模板中进行靶向。DOI:10.1002/cmdc.201402186
文献信息
-
The synthesis of chlorodeoxyhexofuranoid derivatives作者:Haralambos ParolisDOI:10.1016/0008-6215(83)88169-5日期:1983.3hydroxy derivative, whereas treatment of 3 with pyridine gave the 3,5-(cyclic sulfate). Dechlorosulfation of 7 afforded 5,6-dichloro-5,6-dideoxy-1,2-O-isopropylidene-β- l -idofuranose which, on acid hydrolysis, was converted into 3,6-anhydro-5-chloro-5-deoxy- l -idofuranose. 5-Chloro-5-deoxy-α- l -idofuranosidurono-6,3-lactone and 5-chloro-5-deoxy-β- l -idofuranurono-6,3-lactone derivatives were also摘要1,2-O-异亚丙基-α-d-葡萄糖呋喃糖在0°和50°下与硫酰氯反应,得到6-氯-6-脱氧-1,2-O-异亚丙基-α-d-葡萄糖基呋喃糖3 1,5-双(氯硫酸盐)(3)和5,6-二氯-5,6-二脱氧-1,2-O-异亚丙基-β-1-异呋喃糖3-氯硫酸盐(7,未表征)。3的脱氯硫酸得到羟基衍生物,而吡啶处理3则得到3,5-(环硫酸盐)。7的脱硫得到5,6-二氯-5,6-二脱氧-1,2-O-异亚丙基-β-1-异呋喃糖,经酸水解后转化为3,6-脱水-5-氯-5-脱氧-1-氨基呋喃糖。还制备了5-氯-5-脱氧-α-1-偶氮呋喃并呋喃-6,3-内酯和5-氯-5-脱氧-β-1-基呋喃并呋喃-6,3-内酯衍生物。
-
Intramolecular Displacement Reactions Involving Sulfur Leading to the Formation of 3,6‐Thiaanhydro Sugar Derivatives during the Synthesis of 3,5‐Dithio‐glucofuranose作者:Xiaoxiao Liao、Kai Yuan、David CrichDOI:10.1002/ejoc.202101496日期:2022.3.7During a synthetic study targeting 3,5-dithio-glucofuranose, two interesting intramolecular displacement reactions that both generated the 3,6-thiaanhydro structures were discovered. We successfully solved the intramolecular displacement problem by using a 5-S,6-O-isopropylidene protecting group and synthesized the 3,5-dithio-glucofuranose in overall 9 % yields after 14 steps.
-
Synthesis of l-iduronic acid derivatives by epimerisation of anancomeric d-glucuronic acid analogues作者:Neil Baggett、Alan SmithsonDOI:10.1016/s0008-6215(00)81890-x日期:1982.10Abstract Derivatives of l -iduronic acid were prepared by epimerisation of d -glucuronic acid derivatives that were constrained to adopt a conformation having C-6 in an axial position, so that the l -iduronic acid derivatives would be thermodynamically more stable. Methyl 3,5- O -benzylidene-1,2- O -isopropylidene-β- l -idofuranuronate was conveniently obtained in 23% yield by crystallisation from摘要通过对d-葡萄糖醛酸衍生物进行差向异构化,制得了1-iduronic酸的衍生物,这些衍生物必须被限制为采用在轴向位置具有C-6的构象,从而使l-iduronic酸衍生物在热力学上更加稳定。通过从差向异构混合物中结晶,可以方便地以23%的产率获得3,5-O-亚苄基-1,2-O-异亚丙基-β-1-呋喃呋喃甲酸甲酯,并且其特征在于通过还原为相应的醇或通过催化氢化随后进行。通过自发环化得到1,2-O-异亚丙基-β-1-呋喃基呋喃基-6,3-内酯。设计了由d-葡糖呋喃尿酸酯-6,3-内酯分两步合成3,5-O-亚苄基-1,2-O-异亚丙基-α-d-呋喃葡萄糖醛酸甲酯的方法。差向异构的其他候选物,包括其他酯,酰胺和腈,还进行了检查。在所有情况下,通过竞争性分解(可能涉及β消除)都会降低产量。
-
Indium-mediated Reformatsky reaction on lactones: preparation of 2-deoxy-2,2′-dimethyl-3-ulosonic acids作者:Raquel G. SoengasDOI:10.1016/j.tetlet.2009.10.092日期:2010.1Indium-mediated Reformatsky reactions of simple lactones and aldonolactones with ethyl α-bromoisobutyrate allow the synthesis of the corresponding ketals and ulosonic acid esters, respectively, in good yields.
-
[EN] 2-((2S,3S,4R,5R)-5-((S)-3-AMINO-2-HYDROXYPROP-1-YL)-4-METHOXY-3-(PHENYLSULFONYLMETHYL)TETRAHYDROFURAN-2-YL)ACETALDEHYDE DERIVATIVES AND PROCESS FOR THEIR PREPARATION<br/>[FR] DÉRIVÉS DE 2-((2S,3S,4R,5R)-5-((S)-3-AMINO-2-HYDROXYPROP-1-YL)-4-MÉTHOXY-3-(PHÉNYLSULFONYLMÉTHYL)TÉTRAHYDROFURANE-2-YL)ACÉTALDÉHYDE ET PROCÉDÉ POUR LEUR PRÉPARATION申请人:ALPHORA RES INC公开号:WO2013097042A1公开(公告)日:2013-07-04Disclosed is a compound of formula 1, as shown below, where R1, R2, R3, R4, R5, R6 and R7 are as described herein. Also, disclosed is a process for the preparation of compounds of formula 1, and intermediates used therein. The compound of formula 1 can be useful for preparation of halichondrin analogs such as Eribulin.
表征谱图
-
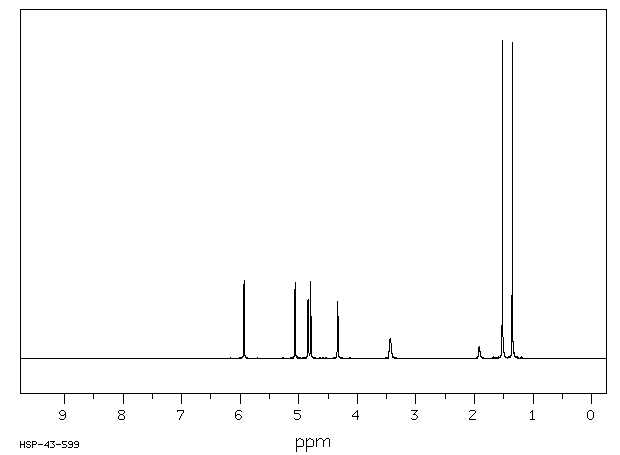
氢谱1HNMR
-
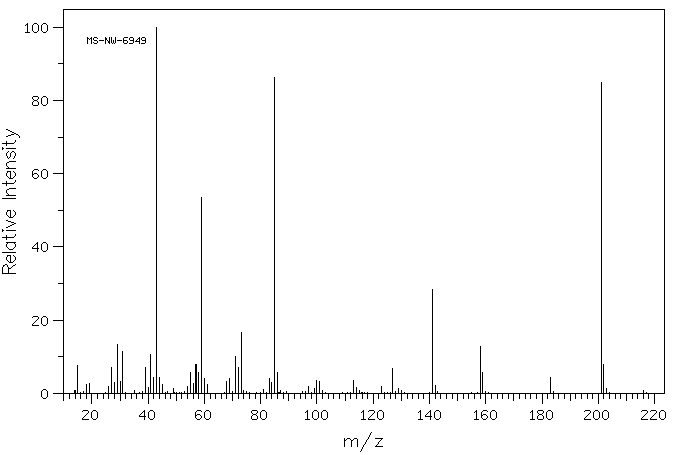
质谱MS
-
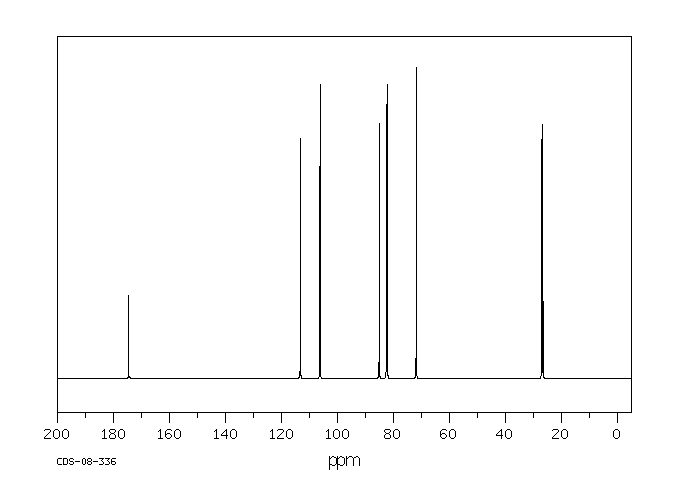
碳谱13CNMR
-
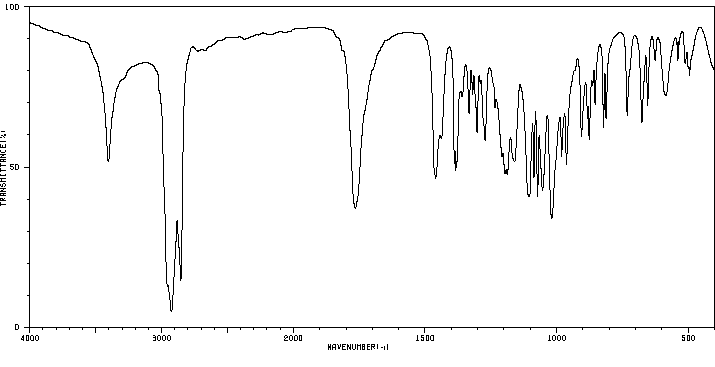
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2,3,3a,6a-四氢呋喃[2,3-b]呋喃
莱克酮
索尼地平
硝酸异山梨酯
溴化二氢6-(联苯基-4-基)-3-氯-12,13-二甲氧基-9,10--7H-异奎并[2,1-d][1,4]苯并二氮卓-8-正离子
星形曲霉毒素
抗坏血酸原 A
异山梨醇二甲基醚
异山梨醇13C65-单酸酯
异山梨醇
失水甘露醇单油酸酯
失水甘露醇单油酸酯
大青素
地瑞那韦中间体1
四氢呋喃[2,3-B]呋喃-2(6AH)-酮
四氢-6a-甲基-呋喃并[2,3-b]呋喃-2(3H)-酮
四氢-6-硫代-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3-酮
去甲斑蝥素
单硝酸异山梨酯杂质C
单-9-十八烯酸1,4:3,6-双脱水-D-甘露醇酯
华北白前甙元B
六氢呋喃并[2,3-b]呋喃-3-醇
六氢呋喃并[2,3-b]呋喃
六氢-呋喃并[2,3-b]呋喃-3-醇
克罗拉滨杂质7
二氯萘
二氢-1,4-二甲基-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3,6(4H)-二酮
二氢-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3,6(4H)-二酮
二氢-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3,6(4H)-二硫酮
乙酸异山梨醇酯
丙氨酸,N-(5-氯-2-羟基苯甲酰)-
N-乙酰基-L-丙氨酰-L-酪氨酸
L-葡糖酸-3,6-内酯
D-葡糖醛酸-γ-内酯丙酮化合物
D-甘露呋喃糖醛酸 gamma-内酯
BISTHFHNS衍生物3
7H,10H-呋喃并[2,3,4-cd]萘并[2,1-e]异苯并呋喃-7-酮,十四氢-10-羟基-1,1,4a-三甲基-,(4aS,4bR,6aR,8aR,10R,10aS,10bR,12aS)-(9CI)
7-氧杂二环[2.2.1]庚-5-烯-2,3-二羧酸酐
6H,9H-苯并[e]呋喃并[2,3,4-cd]异苯并呋喃-6-酮,2,4,4a,5,7,8,10a,10b-八氢-5,5-二甲基-,(4aR,8aR,10aR,10bS)-(9CI)
6-[(1E,3E,5E)-6-[(1R,2R,3R,5R,7R,8R)-7-乙基-2,8-二羟基-1,8-二甲基L-4,6-二氧杂双环[3.3.0]辛-3-基]己-1,3,5-三烯基]-4-甲氧基-5-甲基-吡喃-2-酮
5-单硝酸异山梨酯
5-乙酸异山梨酯
5-乙酸异山梨酯
5-[(4,6-二氯-1,3,5-三嗪-2-基)氨基]-4-羟基-3-[(4-磺酸根-1-萘基)偶氮]萘-2,7-二磺化三钠
5,6-二溴-7-氧杂双环[2.2.1]庚烷-2,3-二甲酸酐
5,5-二甲基-4,8-二氧杂三环[4.2.1.03,7]壬-2-基丙烯酸酯
4-硝基苯并[pqr]四苯-1-醇
4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3-酮
4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3,5-二酮
3-脱氧-14,15-二氢-15-羟基-莸酯素醇