5,5-二甲基海因 | 77-71-4
中文名称
5,5-二甲基海因
中文别名
5-二甲基海因;DM乙内酰脲;5,5-二甲基乙内酰脲;5,5-二甲基海茵;脲丙酮;二甲基海因;DMH;5,5-二甲基咪唑烷基二酮;5,5-二甲乙内酰脲
英文名称
5,5-dimethyl-imidazolidine-2,4-dione
英文别名
5,5-dimethylhydantoin;5,5-dimethylimidazolidine-2,4-dione
CAS
77-71-4
化学式
C5H8N2O2
mdl
MFCD00005266
分子量
128.131
InChiKey
YIROYDNZEPTFOL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:174-177 °C (lit.)
-
沸点:237.54°C (rough estimate)
-
密度:1.2864 (rough estimate)
-
闪点:193°C
-
溶解度:H2O:0.1 g/mL 热的,透明的
-
LogP:-0.480
-
物理描述:Liquid; OtherSolid
-
颜色/状态:White, crystalline solid
-
蒸汽压力:2.8X10-6 mm Hg at 25 °C (est)
-
解离常数:pKa = 9.19
-
稳定性/保质期:
目前主要的工业产品是低黏度(1.5~2.5 Pa·s,25℃)甲基海因环氧树脂。这种树脂黏度低、工艺性能优良,其黏度远低于双酚A型环氧树脂,在无需添加稀释剂的情况下就具备良好的工艺性;热稳定性优异,耐热性高,其绝缘灌封料在180℃下可使用5000小时以上,于130℃下的使用寿命长达40年;耐候性强,在日光或紫外线曝晒下不易发黄和粉化,性能优于双酚A型环氧树脂及丙烯酸树脂涂料,并且其耐盐雾、抗腐蚀性也非常突出。在高电压及超高压环境下表现出色,尤其具有优良的耐电弧性和抗漏电痕迹性;极性强、黏度低,因此对玻璃纤维、碳纤维和多种填料有很好的湿润能力和粘接力,在不降低工艺性的前提下可添加大量填料,从而降低线膨胀系数并降低成本。
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
ADMET
代谢
CD大白鼠,不论雄性和雌性,分别口服给予了14C标记的二甲基硫脲(DMH-14C)100毫克/千克(高剂量)或20毫克/千克(低剂量)的单一剂量。……通过薄层色谱分析,在尿液中发现的唯一主要放射性成分是母体DMH-14C。检测到一个次要代谢物,其量占尿液14C的2.5%。…
CD Rats, /male and female,/ were administered single oral doses of dimethylhydantoin-14C (DMH-14C) at either 100 (high dose) or 20 mg/kg (low dose). ... The parent DMH-14C was the only major radioactive component found in urine by thin layer chromatographic analysis. One minor metabolite was detected, which amounted to 2.5% of the urinary 14C. ...
来源:Hazardous Substances Data Bank (HSDB)
毒理性
描述了一例由摄影印刷涂料液体引起的继发性过敏性接触皮炎的病例...。对混合物的贴片测试反应为阳性,但对包括二甲乙二醇甲醛树脂在内的单个组分为阴性,后者是合理的过敏原。...在这个患者中,需要组分结合才能产生敏感性反应。...之前尚未有关于此产品引起皮炎的报道。
A case of allergic contact dermititis secondary to photographic print coating liquid is described ... . Patch test reactions to the mixture were positive, but negative to the individual components which included dimethylhydantoin formaldehyde resin, the logical allergen. ... In this patient, the combination of components was necessary to yield a sensitivity reaction. ... Dermatitis secondary to this product has not been previously reported.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
立即急救:确保已经进行了充分去污。如果患者停止呼吸,开始人工呼吸,最好使用需求阀复苏器、气囊面罩装置或口袋面罩,按训练进行操作。如有必要,执行心肺复苏。立即用缓慢流动的水冲洗受污染的眼睛。不要催吐。如果发生呕吐,让患者向前倾或将其置于左侧(如果可能,头部向下)以保持呼吸道畅通,防止吸入。保持患者安静,维持正常体温。寻求医疗帮助。/毒物A和B/
Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
基本治疗:建立专利气道(如需要,使用口咽或鼻咽气道)。如有必要,进行吸痰。密切观察呼吸不足的迹象,并在需要时辅助通气。通过非循环呼吸面罩以10至15升/分钟的速度给予氧气。监测肺水肿,并在必要时进行治疗……。监测休克,并在必要时进行治疗……。预防癫痫发作,并在必要时进行治疗……。对于眼睛污染,立即用水冲洗眼睛。在转运过程中,用0.9%的生理盐水(NS)连续冲洗每只眼睛……。不要使用催吐剂。对于摄入,如果患者能吞咽、有强烈的干呕反射且不流口水,则用温水冲洗口腔,并给予5毫升/千克,最多200毫升的水进行稀释……。在去污后,用干燥的无菌敷料覆盖皮肤烧伤……。/毒物A和B/
Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
高级治疗:对于昏迷的患者、严重肺水肿的患者或严重呼吸窘迫的患者,考虑进行口咽或鼻咽气管插管以控制气道。使用带气囊的面罩进行正压通气技术可能有益。考虑对肺水肿进行药物治疗...。对于严重的支气管痉挛,考虑给予β激动剂,如沙丁胺醇...。监测心率和必要时治疗心律失常...。开始静脉输注D5W/SRP:“保持开放”,最小流量/。如果出现低血容量的迹象,使用0.9%生理盐水(NS)或乳酸钠林格氏液。对于伴有低血容量迹象的低血压,谨慎给予液体。注意液体过载的迹象...。使用地西泮或劳拉西泮治疗癫痫...。使用丙美卡因氢氯化物协助眼部冲洗...。/毒物A和B/
Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
中枢神经系统抑制剂
/SIGNS AND SYMPTOMS/ Central nervous system depressant
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
CD大鼠(雄性和雌性)被单次口服给予二甲基乙内酰脲-14C(DMH-14C),剂量为100毫克/千克(高剂量)或20毫克/千克(低剂量)。14C的处置情况被跟踪了七天。... 收集尿液和粪便,并在以下时间段测量14C的量:0-8小时,8-16小时,15-24小时,24-32小时,32-48小时,然后在研究第2天到第5天进行24小时的收集。所有动物在第6天被处死。以下组织被取出并立即冷冻:心脏、肝脏、肾脏、脾脏、腹部脂肪、右腿肌肉、腿骨、性腺和大脑组织。这些组织被测量14C的量。用甲醇清洗笼子内部,并测量洗涤液中的14C。... 从大鼠中回收14C的平均回收率为95%。该化合物被迅速吸收,主要在尿液中排出,仅发生轻微的代谢转化。... 尿液中排出的平均量为剂量的91%。排泄也是迅速的,因为在给药后前24小时内排出了88%。... 在接受高剂量的大鼠中,肾脏和骨骼组织中检测到低水平的14C;然而,所有测量结果均无统计学意义。...
CD Rats, /male and female,/ were administered single oral doses of dimethylhydantoin-14C (DMH-14C) at either 100 (high dose) or 20 mg/kg (low dose). The disposition of the 14C was followed for a seven-day period. ... Urine and feces were collected and measured for the amount of 14C at the following periods: 0 - 8 hours, 8 - 16 hours, 15 - 24 hours, 24 - 32 hours, 32 - 48 hours, then 24 hour collections on Study Days 2 through 5. All animals were killed on Study Day 6. The following tissues were removed and immediately frozen: heart, liver, kidneys, spleen, abdominal fat, right leg muscle, leg bone, gonads and brain tissue. These tissues were measured for amount of 14C. The interior of the cage was washed with methanol and 14C was measured in the wash liquid. ... The average recovery of 14C from the rats was 95%. The compound was absorbed rapidly and was eliminated primarily in the urine with only minor metabolic conversion. ... The average amount eliminated in the urine was 91% of the dose. The elimination also was rapid, as 88% was eliminated in the first 24 hours after dosing. ... In the case of rats receiving the high dose, low levels of 14C were detected in kidney and bone tissues; however all measurements were not significant. ...
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:2,3
-
海关编码:2933990090
-
危险品运输编号:25kgs
-
RTECS号:MU0977000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将产品存放在干燥且密封的环境中保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 5,5-二甲基海因
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 5)
2.2 GHS 标记要素,包括预防性的陈述
象形图 无
警示词 警告
危险申明
H303 吞咽可能有害。
警告申明 无
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C5H8N2O2
分子式
: 128.13 g/mol
分子量
组分 浓度或浓度范围
5,5-Dimethylhydantoin
-
化学文摘登记号(CAS 77-71-4
No.) 201-051-3
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
中枢神经系统抑制, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 174 - 177 °C - lit.
f) 沸点、初沸点和沸程
313 °C 在 1,012.10 hPa
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
0.0000038 hPa 在 25 °C
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
140 g/l 在 20 °C - 完全溶解
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: -0.475 在 22.4 °C
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 雄性和雌性 - 5,000 mg/kg
半数致死剂量 (LD50) 经皮 - 兔子 - 雄性和雌性 - > 20,000 mg/kg
皮肤刺激或腐蚀
皮肤 - 兔子 - 无皮肤刺激 - 4 h - 经济合作与发展组织的试验指南404
眼睛刺激或腐蚀
眼睛 - 兔子 - 无眼睛刺激 - 经济合作与发展组织的试验指南405
呼吸道或皮肤过敏
豚鼠封闭斑贴试验 - 豚鼠 - 不引起皮肤过敏。 - 经济合作与发展组织的试验指南406
生殖细胞致突变性
细胞突变性-体外试验 - 小鼠 - 淋巴细胞 - 有或没有代谢活化作用 - 阴性
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
中枢神经系统抑制, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
反复染毒毒性 - 大鼠 - 雄性和雌性 - 经口 - 未观察到有害效果的水平 - 300 mg/kg
化学物质毒性作用登记: MU0977000
模块 12. 生态学资料
12.1 生态毒性
对鱼类的毒性 静态试验 半数致死浓度(LC50) - 虹鳟 (红鳟鱼) - > 972.2 mg/l - 96 h
对水蚤和其他水生无脊 静态试验 半数效应浓度(EC50) - 大型蚤 (水蚤) - 6,200 mg/l - 48 h
椎动物的毒性
对藻类的毒性 生长抑制 半数效应浓度(EC50) - 近头状伪蹄形藻 (绿藻) - > 1,000 mg/l - 96 h
方法: 经济合作和发展组织的试验指导书201
细菌毒性 呼吸抑制 半数效应浓度(EC50) - 污泥处理 - > 1,000 mg/l - 3 h
方法: 经济合作和发展组织的试验指导书209
12.2 持久性和降解性
生物降解能力 好氧的 - 接触时间 28 d
结果: 88 % - 易生物降解。
方法: 经合组织(OECD )测试指南 301B
12.3 潜在的生物累积性
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
5,5-二甲基海因是一种广泛应用于有机合成、医药、塑料、食品、化妆品和农药等领域的有机合成中间体,属于具有一定刚性骨架的杂环化合物。可通过取代反应制备一系列有价值的衍生物,例如二溴海因、二氯海因等。
应用5,5-二甲基海因,又名5,5-二甲基乙内酰脲(DMH),是一种用途广泛的有机合成中间体。它可以通过取代反应生成多种有价值的衍生物,如二溴海因和二氯海因等,广泛应用于医药、农药、塑料和化妆品等生产领域的精细化学品。
制备制备5,5-二甲基海因的过程包括以下几个步骤:
- 将600~800份碳酸氢铵与500~700份丙酮氰醇加水搅拌充分溶解,加热至10℃时密闭。
- 向上述物质中通入氨气30~50份,并缓慢加温至50~65℃。在恒温下持续搅拌1~2小时。
- 将上述混合物加热至75~85℃,引风30~50分钟,冷却至室温后过滤,即可得到二甲基海因粗品和母液。
- 将950~1150份二甲基海因粗品投放到150~300份水中以及700~900份母液混合物中。边投放边搅拌,并加热至80~98℃,加入活性碳3~8份进行脱色20~40分钟,过滤去除活性碳,再冷却结晶、离心过滤和烘干,即可得到二甲基海因成品。
5,5-二甲基海因为白色棱状体结晶或结晶性粉末。熔点为175℃。该化合物可溶于水、己醇、乙酸乙酯和二甲醚中,微溶于异丙酮、丙酮和甲乙酮中,而不溶于脂肪烃和三氯乙烯。无臭且能升华,呈酸性。
用途主要用作杀菌消毒剂及环氧树脂和氨基酸的原料。其衍生物如1,3-二氯-5,5-二甲基乙内酰脲可用作氯化剂、消毒杀菌剂;而3-羟甲基-5,5-二甲基乙内酰脲则可用作甲醛化剂、防腐剂等。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-溴-5,5-二甲基海因 1,3-dibromo-5,5-dimethyl-hydantoin 7072-23-3 C5H7BrN2O2 207.027 羟甲基-5,5-二甲基咪唑烷-2,4-二酮 1-(hydroxymethyl)-5,5-dimethyl hydantoin 116-25-6 C6H10N2O3 158.157 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,5,5-三甲基咪唑烷-2,4-二酮 3,5,5-trimethyl-2,4-imidazolidinedione 6345-19-3 C6H10N2O2 142.158 1,5,5-三甲基海因 1,5,5-trimethylhydantoin 6851-81-6 C6H10N2O2 142.158 5-(氯甲基)-5-甲基咪唑烷-2,4-二酮 5-chloromethyl-5-methylhydantoin 33109-56-7 C5H7ClN2O2 162.576 3-氯-5,5-二甲基海因 3-chloro-5,5-dimethyl-imidazolidine-2,4-dione 34979-51-6 C5H7ClN2O2 162.576 3-氨基-5,5-二甲基咪唑烷-2,4-二酮 3-amino-5,5'-dimethylhydantoin 1123-44-0 C5H9N3O2 143.145 1-氯-5,5-二甲基咪唑烷-2,4-二酮 1-chloro-5,5-dimenthylhydantoin 6921-17-1 C5H7ClN2O2 162.576 3-溴-5,5-二甲基海因 3-bromo-5,5-dimethylhydantoin 58402-65-6 C5H7BrN2O2 207.027 1-溴-5,5-二甲基海因 1,3-dibromo-5,5-dimethyl-hydantoin 7072-23-3 C5H7BrN2O2 207.027 1,3,5,5-四甲基咪唑烷-2,4-二酮 1,3-dimethyl-5,5-dimethyl hydantoin 15414-89-8 C7H12N2O2 156.184 3-乙烯基-5,5-二甲基咪唑烷-2,4-二酮 3-Vinyl-5,5-dimethylhydantoin 31787-67-4 C7H10N2O2 154.169 羟甲基-5,5-二甲基咪唑烷-2,4-二酮 1-(hydroxymethyl)-5,5-dimethyl hydantoin 116-25-6 C6H10N2O3 158.157 1-(2-氨基乙基)-5,5-二甲基-2,4-咪唑烷二酮 1-(2-aminoethyl)-5,5-dimethyl-imidazolidine-2,4-dione 893433-60-8 C7H13N3O2 171.199 —— 1-(2-Hydroxyethyl)-5,5-dimethylimidazolidine-2,4-dione 88280-55-1 C7H12N2O3 172.18 —— 1-(Prop-2-enyl)-4,4-dimethyl-2,5-dioxoimidazolidine 3366-92-5 C8H12N2O2 168.195 —— 1-cyano-3-(4,4-dimethyl-2,5-dioxoimidazolidin-1-yl)propane 27430-50-8 C8H11N3O2 181.194 —— 3-(2-bromoethyl)-5,5-dimethylimidazolidine-2,4-dione 13272-29-2 C7H11BrN2O2 235.081 3-(2-羟基乙基)-5,5-二甲基咪唑烷-2,4-二酮 3-(2-hydroxypropyl)-5,5-dimethylhydantoin 29071-93-0 C7H12N2O3 172.184 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:高效非对映选择性合成4- N-甲基甲酰胺基特灵(GV129606),一种有效的抗菌剂摘要:在本文中,报道了4- N-甲基甲酰胺基trinem 3的高度非对映选择性合成。与以前使用的路线相比,该路线具有优势,即更高的总收率,坚固性,避免了有毒试剂。大多数化合物通过沉淀分离,因此减少了色谱分离次数。通过使用清除剂树脂的新方法获得了4- N-甲基氨基三苯甲基11到GV129606 3的有效转化。本文提出的途径可以为早期开发研究准备所需的材料,并证明环己烯基氮杂环丁酮12的多功能性 在合成4-取代的triems。DOI:10.1016/s0040-4020(00)00414-2
-
作为产物:描述:参考文献:名称:高效非对映选择性合成4- N-甲基甲酰胺基特灵(GV129606),一种有效的抗菌剂摘要:在本文中,报道了4- N-甲基甲酰胺基trinem 3的高度非对映选择性合成。与以前使用的路线相比,该路线具有优势,即更高的总收率,坚固性,避免了有毒试剂。大多数化合物通过沉淀分离,因此减少了色谱分离次数。通过使用清除剂树脂的新方法获得了4- N-甲基氨基三苯甲基11到GV129606 3的有效转化。本文提出的途径可以为早期开发研究准备所需的材料,并证明环己烯基氮杂环丁酮12的多功能性 在合成4-取代的triems。DOI:10.1016/s0040-4020(00)00414-2
-
作为试剂:描述:5-甲基-2-硝基苯甲酸 在 偶氮二异丁腈 、 5%-palladium/activated carbon 、 5,5-二甲基海因 、 氢气 、 sodium formate 、 乙酸酐 、 sodium hydroxide 作用下, 以 甲醇 、 水 、 氯苯 为溶剂, 反应 21.0h, 生成 2-氨基-5-[[甲酰基(甲基)氨基]甲基]苯甲酸参考文献:名称:SULFONAMIDE DERIVATIVE AND PHARMACEUTICAL USE THEREOF摘要:提供的是一种磺酰胺衍生物,其具有以下通式(1)表示,并具有对α4整合素抑制效果的高选择性,对α4β1影响小,对α4β7影响大,或其药用可接受盐(在通式(1)中,A、B、D、E、R 41 和a至h如描述中所述)。公开号:US20160244451A1
文献信息
-
Development of New Hydroxamate Matrix Metalloproteinase Inhibitors Derived from Functionalized 4-Aminoprolines作者:Michael G. Natchus、Roger G. Bookland、Biswanath De、Neil G. Almstead、Stanislaw Pikul、Michael J. Janusz、Sandra A. Heitmeyer、Erin B. Hookfin、Lily C. Hsieh、Martin E. Dowty、Charles R. Dietsch、Vikram S. Patel、Susan M. Garver、Fei Gu、Matthew E. Pokross、Glen E. Mieling、Timothy R. Baker、David J. Foltz、Sean X. Peng、David M. Bornes、Michael J. Strojnowski、Yetunde O. TaiwoDOI:10.1021/jm000246e日期:2000.12.1activity with sub-nanomolar potency for some enzymes. Modifications of the P1' portion of the molecule played a key role in affecting both potency and selectivity within the MMP family. Longer-chain aliphatic substituents in this region of the molecule tended to increase potency for MMP-3 and decrease potency for MMP-1, as exemplified by compounds 48-50, while aromatic substituents, as in compound 52, generated从氨基脯氨酸支架制备了一系列异羟肟酸酯,并测试了其作为基质金属蛋白酶(MMP)抑制剂的功效。据报道该系列的详细SAR是针对MMP家族中的5种酶,许多抑制剂(例如化合物47)具有广谱活性,某些酶具有亚纳摩尔的效力。分子P1'部分的修饰在影响MMP家族内的效能和选择性方面起着关键作用。如化合物48-50所示,在分子的该区域中的长链脂族取代基倾向于增加对MMP-3的效力而降低对MMP-1的效力,而如化合物52中的芳族取代基产生广谱抑制作用。该数据基于X射线晶体数据也被合理化。虽然体外经口吸收似乎不太可预测,但随着更长和更多亲水性取代基的出现,吸收率往往会降低。最后,使用骨关节炎的大鼠模型评估这些化合物的功效,并在它们的药代动力学和体内功效之间建立了直接联系。
-
Water Phase, Room Temperature, Ligand-Free Suzuki-Miyaura Cross-Coupling: A Green Gateway to Aryl Ketones by C-N Bond Cleavage作者:Yuqi Zhang、Zijia Wang、Zhao Tang、Zhongfeng Luo、Hongxiang Wu、Tingting Liu、Yulin Zhu、Zhuo ZengDOI:10.1002/ejoc.201901730日期:2020.3.22An environmentally friendly palladium‐catalyzed Suzuki–Miyaura coupling reaction of twisted amides with potassium aryl trifluoroborates has been developed, generating ketones under pure water phase, room temperature, and ligand‐free condition.
-
[EN] CORTISTATIN ANALOGUES AND SYNTHESES AND USES THEREOF<br/>[FR] ANALOGUES DE CORTISTATINE ET SYNTHÈSES ET UTILISATIONS ASSOCIÉES申请人:HARVARD COLLEGE公开号:WO2015100420A1公开(公告)日:2015-07-02Provided herein are compounds of Formula (A), (B), (C), (D) and (E), pharmaceutically acceptable salts, quaternary amine salts, and N-oxides thereof, and pharmaceutical compositions thereof. Compounds of Formula (A), (B), (C), (D), and (E) are contemplated useful as therapeutics for treating a wide variety of conditions, e.g., including but not limited to, conditions associated with angiogenesis and with CDK8 and/or CDK19 kinase activity. Further provided are methods of inhibiting CDK8 and/or CDK19 kinase activity, methods of modulating the β-catenin pathway, methods of modulating STAT1 activity, methods of modulating the TGFβ/BMP pathway, methods of modulating HIF-1 -alpha activity in a cell, and methods of increasing BIM expression to induce apoptosis, using a compound of Formula (A), (B), (C), (D), or (E). Further provided are CDK8 and CDK19 point mutants and methods of use thereof.本文提供了公式(A)、(B)、(C)、(D)和(E)的化合物、药用可接受的盐、季铵盐和N-氧化物,以及它们的药物组合物。公式(A)、(B)、(C)、(D)和(E)的化合物被认为是有用的治疗剂,用于治疗各种疾病,例如,包括但不限于,与血管生成和与CDK8和/或CDK19激酶活性相关的疾病。还提供了抑制CDK8和/或CDK19激酶活性的方法,调节β-连环蛋白途径的方法,调节STAT1活性的方法,调节TGFβ/BMP途径的方法,调节细胞中HIF-1α活性的方法,以及使用公式(A)、(B)、(C)、(D)或(E)的化合物增加BIM表达以诱导凋亡的方法。还提供了CDK8和CDK19点突变体及其使用方法。
-
Catalytic Halogen Bond Activation in the Benzylic C–H Bond Iodination with Iodohydantoins作者:Sascha H. Combe、Abolfazl Hosseini、Lijuan Song、Heike Hausmann、Peter R. SchreinerDOI:10.1021/acs.orglett.7b03034日期:2017.11.17This letter presents the side-chain iodination of electron-deficient benzylic hydrocarbons at rt using N-hydroxyphthalimide (NHPI) as radical initiator and 1,3-diiodo-5,5-dimethylhydantoin and 3-iodo-1,5,5-trimethylhydantoin (3-ITMH) as iodine source. Addition of a carboxylic acid increased the reactivity due to complex formation with and activation of 3-ITMH by proton transfer and halogen bond formation
-
Reverse hydroxamate inhibitors of matrix metalloproteinases申请人:Abbott Laboratories公开号:US06294573B1公开(公告)日:2001-09-25Compounds having the formula are matrix metalloproteinase inhibitors. Also disclosed are matrix metalloproteinase-inhibiting compositions and methods of inhibiting matrix metalloproteinase in a mammal.
表征谱图
-
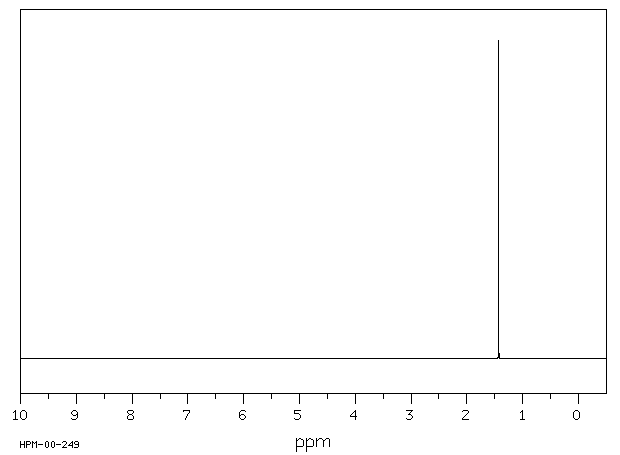
氢谱1HNMR
-
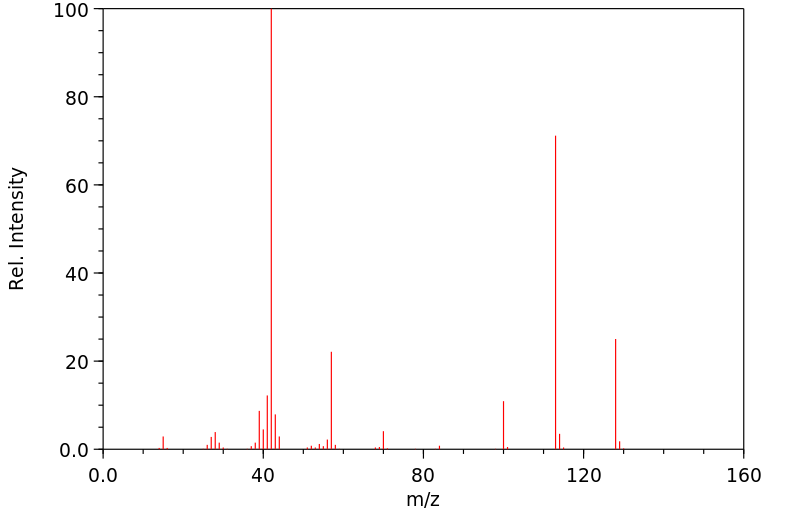
质谱MS
-
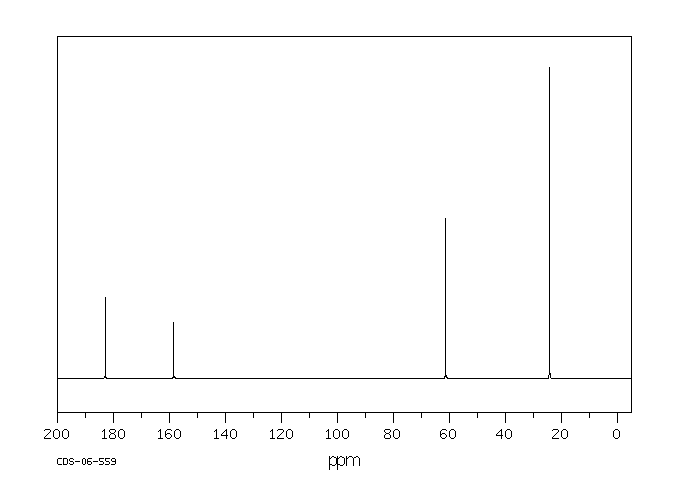
碳谱13CNMR
-
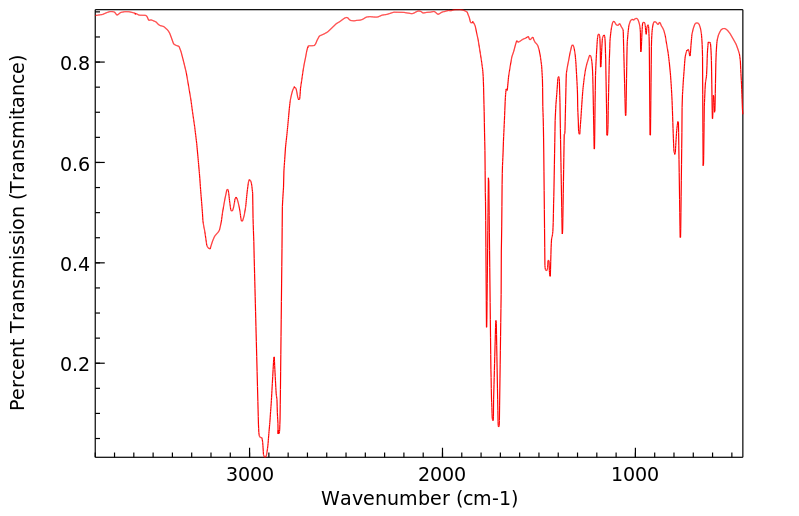
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)