Β,2-二硝基苯乙烯 | 5670-67-7
中文名称
Β,2-二硝基苯乙烯
中文别名
——
英文名称
(E)-1-nitro-2-(2-nitrovinyl)benzene
英文别名
1-nitro-2-((E)-2-nitrovinyl)benzene;1-Nitro-2-(2-nitrovinyl)benzene;1-nitro-2-[(E)-2-nitroethenyl]benzene
CAS
5670-67-7;3156-39-6
化学式
C8H6N2O4
mdl
MFCD00052066
分子量
194.147
InChiKey
INIQBBVYBCGEIX-AATRIKPKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:106-108 °C(lit.)
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:14
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:91.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:β-硝基苯乙烯作为合成 β-芳基-γ-内酰胺和 2-氧代-1,2-二氢喹啉衍生物的有价值的前体摘要:我们成功地展示了使用 β-硝基苯乙烯合成 β-芳基-γ-内酰胺和 2-氧代-1,2-二氢喹啉衍生物的两种不同反应途径。反应策略涉及迈克尔加成,然后是还原和环化反应。迈克尔加合物含有两个不同的硝基,如一个在芳环上,另一个在侧链上,选择性地采取涉及芳基上的硝基的路径,形成2-氧代-1,2-二氢喹啉衍生物作为环化产物。仅在侧链上具有硝基的迈克尔加合物形成β-芳基-γ-内酰胺衍生物作为环化产物。该方法具有可合成的克级产品的可靠性和可扩展性,具有吸引力。这些杂环化合物可以利用它们的生物活性。DOI:10.1002/jhet.4446
-
作为产物:描述:2-nitrocinnamic acid 在 三甲基氯硅烷 、 copper(II) nitrate trihydrate 作用下, 以 乙腈 为溶剂, 反应 2.0h, 以60%的产率得到Β,2-二硝基苯乙烯参考文献:名称:氯/溴三甲基硅烷-铜(NO 3)2 ·3H 2 ○:安全有效的试剂系统的脱羧本位-nitration和肉桂酸二溴化摘要:揭示了卤代三甲基硅烷-硝酸盐混合物的进一步合成潜力。TMSX-Cu构成(NO的混合物3)2 ·3H 2 O系统被发现是两者的脱羧硝化的有效试剂系统(本位-nitration)当X = Cl和的肉桂酸,与X = Br的二溴化,在温和的条件下。该反应是安全且简单的,可以在相对较短的时间内以高收率高产率获得相应的产物(E)-β-硝基苯乙烯和抗-2,3-二溴-3-苯基丙酸。可以避免使用有害和有毒的硝化系统,例如硝酸硝酸乙酰酯和溴化剂,例如分子溴。DOI:10.1016/j.tetlet.2017.06.017
文献信息
-
Asymmetric Michael addition reactions catalyzed by a novel upper-rim functionalized calix[4]squaramide organocatalyst作者:Ke Yang、Zhiyan Ma、Hong-Xiao Tong、Xiao-Qiang Sun、Xiao-Yu Hu、Zheng-Yi LiDOI:10.1016/j.cclet.2020.02.057日期:2020.12Abstract A novel upper-rim functionalized calix[4]squaramide organocatalyst bearing bis-squaramide and cyclohexanediamine scaffolds was designed and prepared to catalyse a serial of asymmetric Michael addition of 1,3-dicarbonyl compounds to α,β-unsaturated carbonyl compounds in high yields (up to 99 %) and good to excellent enantiomeric excesses (up to 99% ee). The comparative experiments indicated
-
Prolinal dithioacetals: Highly efficient organocatalysts for the direct nitro-Michael additions in both organic and aqueous media作者:Tanmay Mandal、Wen Kuo、Matthew Su、Kartick Bhowmick、John C.-G. ZhaoDOI:10.1016/j.tet.2017.10.008日期:2017.11Some novel prolinal dithioacetal derivatives have been synthesized and applied as the organocatalysts for the direct Michael addition of ketones and aldehydes to nitroalkenes. High enantioselectivities and diastereoselectivities have been obtained in both organic and aqueous media (dichloromethane, water, or brine).
-
Visible-light-enabled denitrative carboxylation of β-nitrostyrenes: a direct photocatalytic approach to cinnamic acids作者:Shubhangi Tripathi、Lal Dhar S. YadavDOI:10.1039/c7nj04578f日期:——The first workable application of β-nitrostyrenes and CBr4 as coupling partners for a highly stereoselective synthesis of (E)-cinnamic acids under visible light photoredox catalysis is reported. The reaction involves a radical denitrative tribromomethylation/hydrolysis cascade to afford (E)-cinnamic acids in excellent yields at room temperature in a one-pot procedure. Moreover, the implementation of
-
Preparation of Pyrrolidine-Based PDE4 Inhibitors via Enantioselective Conjugate Addition of α-Substituted Malonates to Aromatic Nitroalkenes作者:Paul J. Nichols、John A. DeMattei、Bradley R. Barnett、Nicole A. LeFur、Tsung-Hsun Chuang、Anthony D. Piscopio、Kevin KochDOI:10.1021/ol060398p日期:2006.3.1[reaction: see text] The enantioselective conjugate addition of alpha-substituted malonates to aromatic nitroalkenes generates a stereocenter at the carbon bearing the aromatic group and an adjacent prochiral center from the alpha-substituted malonate. Nitro reduction followed by diastereoselective cyclization provides pyrrolidinones with two contiguous stereocenters, one of which is quaternary. This
-
4-Trifluoromethanesulfonamidyl prolinol tert-butyldiphenylsilyl ether as a highly efficient bifunctional organocatalyst for Michael addition of ketones and aldehydes to nitroolefins作者:Chao Wang、Chun Yu、Changlu Liu、Yungui PengDOI:10.1016/j.tetlet.2009.02.211日期:2009.54-Trifluoromethanesulfonamidyl prolinol tert-butyldiphenylsilyl ether bifunctional organocatalyst 3a is a highly efficient catalyst for the asymmetric Michael addition reactions of ketones and aldehydes to nitrostyrenes, leading to syn-selective adducts with excellent yields (>99%), high diastereoselectivities (up to 99:1 dr) and excellent enantioselectivities (up to 99% ee). Control experiments suggested that the
表征谱图
-
氢谱1HNMR
-
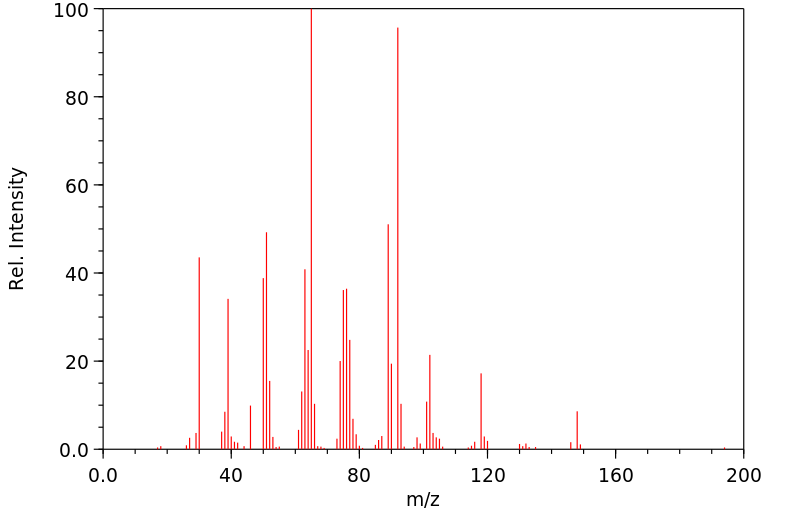
质谱MS
-
碳谱13CNMR
-
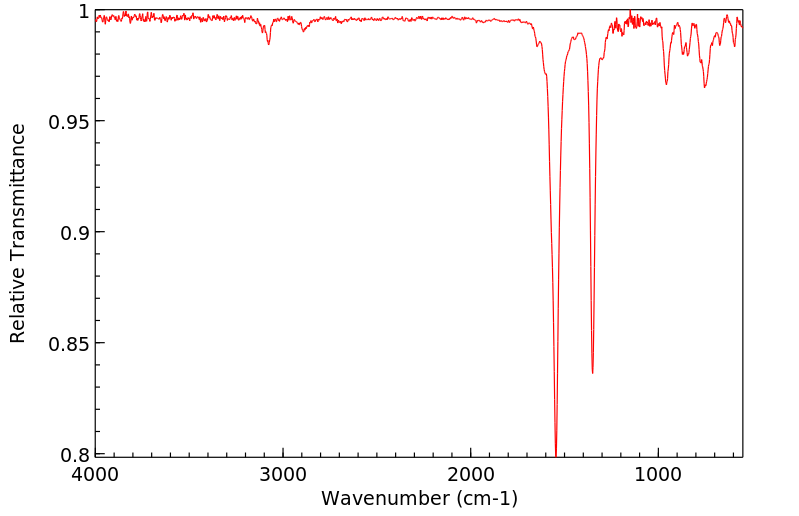
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫